Science > Chemistry > Electrochemistry > Primary Electrochemical Cells
Types of Electrochemical Cell:
An electrochemical cell is a device which is used to generate electrical energy at the expense of spontaneous oxidation-reduction reaction. An electrochemical cell is also known as a galvanic cell or a voltaic cell. In an electrochemical cell, chemical energy is converted into electrical energy. There are two types of voltaic or galvanic cells used to get an electric current of low voltage: a) Primary cells and b)Secondary cells
Primary Cell:
A cell in which electrical energy is generated within the cell itself is called a primary cell. e.g. Dry cell, Daniell cell. Such a cell cannot be reused.
Secondary Cell:
A cell in which electrical energy is not generated within the cell itself but it previously stored in it from an external source is called the secondary cell. e.g. Lead accumulator. Secondary cells can be reused.
Primary Cells:
Daniel cell:
Principle:
Due to the spontaneous redox reaction, electrical energy is generated within the cell itself. So Daniel cell is a primary voltaic cell. When an opposing voltage slightly greater than the cell voltage is applied, the cell reaction is reversed so Daniel cell is a reversible cell. Thus Daniel cell is a primary reversible electrochemical cell.
Construction:
Daniel cell consists of two electrodes or two half cells i.e. zinc half-cell and copper half-cell. Daniel cell with porous partition consists of a vessel which is divided into two compartments
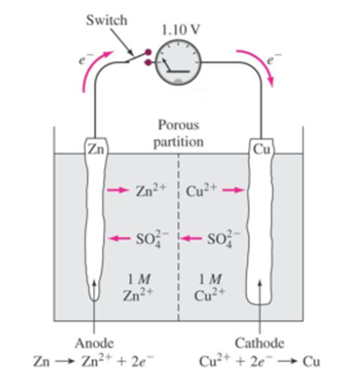
In one compartment, a solution of zinc sulphate ZnSO4 is placed in which a zinc rod is dipped. This rod acts as a negative electrode or anode. This is known as the negative half cell or zinc half cell. The other compartment is filled with copper sulphate CuSO4 solution. In this solution, a copper rod is dipped which acts as a positive electrode cathode. This is known as a positive half cell or copper half cell.
The two half-cells are connected externally by a conducting wire and internally by means of a porous partition or salt bridge or porous pot. The porous partition allows the diffusion of ions through it but prevents excessive mixing of the two solutions.
Representation of the Cell:
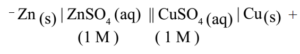
Working of the cell:
As long as the circuit is closed, the following reactions take place. Oxidation takes place at zinc half cell and reduction takes place at the copper half cell. Zinc rod acts as anode i.e. -ve electrode while copper rod acts as cathode i.e. + ve electrode
Cell reactions:
At anode (Zn) or – ve electrode:
Zinc atoms from rod enter the solution forming Zn ions ( Zn++) leaving behind 2 electrons on the rod.
Zn → Zn++ + 2 e– (oxidation)
At cathode (Cu) or + ve electrode:
Cu++ ions from CuSO4 solution acquire 2 electrons from the copper rod and during this process get deposited on the copper electrode in the form of Cu atoms.
Cd++ + 2 e– → Cd (reduction)
Net cell reaction:
Zn + Cu++ → Zn++ + Cu (redox)
Both the oxidation and reduction reaction take place simultaneously and separately. Due to oxidation, the zinc rod becomes electron-rich and due to the reduction copper rod becomes electron deficient. Thus there is a flow of electrons from electron-rich zinc rod to the electron-deficient copper rod through an external circuit which constitutes the electric current.
The movements of sulphate (SO4– –) ions from copper sulphate to zinc sulphate through the salt bridge or porous pot completes the Internal circuit and maintain electrical neutrality of the electrolyte.
The size of the zinc rod reduces because it dissolves in ZnSO4 solution as zinc ions while the size of copper rod increases because of Cu++ in the CuSO4 solution discharge and copper deposits on the rod. The concentration of ZnSO4 solution increases while the concentration of CuSO4 solution decreases. The solutions in both the compartments remain electrically neutral.
E. M. F. of Daniel cell:
If the concentrations of zinc sulphate and copper sulphate solutions are 1 M each then the e.m.f. of Daniel cell is about 1.1 volt.
Reversibility of Daniel cell:
If an external opposing e.m.f. slightly greater than 1.1 V is applied then the reverse cell reaction takes place.
Zn++ + Cu → Zn + Cu++ (redox)
Now, reduction takes place at the zinc electrode and oxidation takes place at the copper electrode. Thus Daniel cell is a reversible cell.
Notes:
Explanation of why there is a gradual decrease in e.m.f. of Daniel cell
EoCell = Eo(ox/cathode) – E(ox/anode)
EoCell = Eo(ox/Zn) – Eo(ox/Cu)
EoCell = 0.76 – (- 0.34)
EoCell = 0.76 + 0.34
EoCell = 1.1 V
As the concentration of Zn++ ions goes on increasing in zinc half cell, the oxidation potential of zinc electrode goes on decreasing. As the concentration of Cu++ ions go on decreasing in the copper half cell, the reduction potential of copper electrode goes on decreasing.
Thus the quantity EoCell = Eo(ox/Zn) + Eo(red/Cu) decreases.
Daniel Cell with Porous Pot:
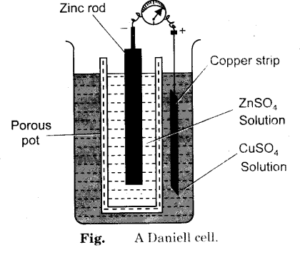
Daniel Cell with Copper Vessel as Cathode:

Daniel Cell with Salt Bridge:

Dry Cell:
The dry cell is actually a modification of the Leclanche cell. A dry cell is a primary cell because electrical energy is generated within the cell itself. It is an irreversible cell as cell reaction doesn’t reverse if higher external emf is applied to it. Since it doesn’t contain any liquid it is called the dry cell. It is very convenient to carry.
Construction:

A dry cell consists of an outer zinc vessel (can) which works as an anode or negative electrode. In the centre of the zinc vessel, there is a stout graphite rod surrounded by compressed is an aqueous paste of MnO2, carbon powder and ammonium chloride. This compressed solid is placed at the centre of the vessel.
The gap between zinc can and the compressed mixture is filled up with a jelly paste prepared from zinc chloride, ammonium chloride, and starch. Starch jelly helps to keep ammonium chloride moist for a long time. These Two different pastes are separated by means of a cotton partition.
The top of the can is sealed with sealing wax or resin or plastic to avoid drying of the paste. The whole-cell is covered with insulating safety cover.
Working:
On connecting carbon (+) and zinc (-) terminals through an external circuit, the cell starts working. When cell functions, oxidation takes place at zinc vessel and reduction takes place at graphite.
Reactions at negative electrode (Zn) :
Zn → Zn++ + 2 e– (oxidation)
Reactions at positive electrode (C) :
2 MnO2 + 2 NH4+ + 2e– → Mn2O3 + 2NH3(g) + H2O(l) (reduction)
Net Cell Reaction:
The overall reaction is,
Zn + 2 MnO2 + 2 NH4+ → Zn++ +Mn2O3 + 2NH3(g) + H2O(l)
Thus zinc is consumed continuously during working and holes are formed in the container which results in the leakage of the cell contents.
Side Reaction :
Zn++ + NH3 → [Zn(NH3]++
The EMF of Dry Cell:
The EMF of this cell is 1.5 V.
Reversibility of Cell:
A dry cell is a non-reversible cell.
Uses of the dry cell.
- The dry cell is used in torches, calculators, and toys.
- Dry cells are used in flashlights.
- They are extensively used in many electronic appliances like clocks, transistor radios, tape recorders, calculators, etc.
Disadvantages of a Dry Cell:
- The acidic ammonium chloride corrodes the zinc container and thus the dry cell does not have a long time.
- Zinc is consumed continuously during working and holes are formed in the container which results in the leakage of the cell contents.
- This cell cannot be recharged by applying an external potential. This is because Zn++ ions and NH3 molecule react each other to form a complex [Zn(NH3)]++ and they are not available for the reverse reaction. Therefore the dry cell is an irreversible cell.
Irreversibility of the dry cell:
During discharging of a dry cell following reaction takes place.
Zn → Zn++ + 2 e– (oxidation)
Thus zinc of vessel dissolves in the surrounding electrolyte forming zinc ions. These Zn++ ions and NH3 molecule react each other to form complex {Zn(NH3)]++
Thus if we apply an external e.m.f. zinc ions are not available for the reverse reaction. Hence instead of the reduction of zinc, the reduction of ammonium ion takes place as follows.
2 NH4+ +2 e– → 2NH3(g) + H2 (reduction)
Hence due to the absence of reverse reaction. The cell is irreversible.
Button Cell:

Construction:
- Anode can: Usually it contains zinc metal and electrolyte mixture (zinc powder + mercury). This forms the negative terminal of the cell.
- Cathode can: It contains the cathode material/electrolyte mixture (ZnO + KOH). This forms the positive terminal of the cell.
- Separator: It contains porous material with electrolyte.
Working:
On connecting cathode (+) and anode (-) terminals through an external circuit, the cell starts working.
Cell Reactions:
Reactions at anode:
Zn + 2OH– → ZnO(s) + H2O(l) + 2 e– (oxidation)
Reactions at cathode:
HgO(s) + H2O(l) + 2 e– → Hg(l) + 2OH– (reduction)
Net Cell Reaction:
The overall reaction is,
Zn + HgO(s) → ZnO(s) + Hg(l)
The EMF of cell:
The EMF of this cell is 1.35 V. Practically this e.m.f. remains constant because no ions are involved whose concentration can change during use.
Reversibility of Cell:
The button cell is a non-reversible cell.
Uses of the button cell.
- It is small in size hence can be used in digital and electronic instruments like watches, computers, hearing aids, etc.
- The extremely constant voltage over its useful life. Suitable for low drain and intermittent high drain applications.
- It has a long shelf life – up to 3 years.
Disadvantages of the Button Cell:
- It contains mercury, which in certain forms is highly toxic to humans and animals.
Fuel cells:
Fuel cells are galvanic cells in which fuel, in the form of gases, which constitute electrode material are constantly supplied to the cell and electrochemical reaction takes place to produce electricity. The galvanic cells in which the energy of the combustion of fuels is directly converted into electrical energy are called fuel cells.
As it is a direct method to convert chemical energy into electrical energy, the cell has an efficiency of 70%. In another method by burning fossil fuel like coal, coke, petrol, diesel heat is generated, which is used to raise steam, which in turn used to rotate generators to produce electricity. This method has an efficiency of 40 % only.
Construction of Fuel Cell:

It consists of a cylindrical container of alkali-resistant material. It is divided into three compartments. The middle compartment contains a hot concentrated solution of KOH. Pure and dry hydrogen gas enters the anodic compartment from one side and pure dry oxygen enters the cathodic compartment from the other side.
The anode is a porous carbon electrode impregnated with platinum or palladium which acts as a catalyst and provides electrical contact. The cathode is also a porous carbon electrode impregnated with cobalt oxide, platinum or silver which acts as a catalyst and provides electrical contact.
Working of Fuel Cell:
Anode reaction: Hydrogen gas under high pressure diffuses through porous carbon anode
2 H2(g) + 4OH–(aq)→ 4H2O(l) + 4e–
Cathode reaction: The electron generated at anode reach cathode through external circuit and oxygen adsorbed on the surface of cathode get reduced to hydroxyl ions.
O2(g) + H2O(l) + 4e– → 4OH–
Thus the net reaction of the cell is
2 H2(g) + O2(g) → 2H2O(l)
Thus water is the by-product of the reaction. These cells do not pollute the atmosphere.
E.M.F. of a Cell:
EoCell = Eo(ox/cathode) – E(ox/anode)
EoCell = 0.83 + 0.40
EoCell = 1.23 V
Advantages of Fuel Cells:
- The reacting substances are continuously supplied to the electrodes. Hence unlike conventional cells, the fuel cells do not have to be discharged when the chemicals are consumed.
- Thus water is the by-product of the reaction. These cells do not pollute the atmosphere.
- As it is a direct method to convert chemical energy into electrical energy, the cell has an efficiency of 70%.
Drawbacks of Fuel Cells:
- The calculated cell voltage is 1.23 V. Due to the reversibility of the reaction the cell voltage is less than 1.23 V.
- Hydrogen gas is hazardous and its production is expensive.
Uses Fuel Cells:
- These cells have been used in automobiles on an experimental basis.
- These cells are used in space shuttle and space stations to produce electricity and water for consumption for onboard astronauts.
- They may be used in hospitals, hotels, and homes for the production of electricity.
Previous Topic: Electrolysis and its applications
Next Topic: Secondary Electrochemical Cells