Science > Chemistry > Electrochemistry > Secondary Electrochemical Cells
In this article, we shall study the construction and working of lead accumulator, and Ni-Cd cells.
Lead Accumulator:
A lead accumulator is a secondary cell because electrical energy is not generated within the cell itself but it is previously stored in it from the external source. It is reversible cell because cell reactions are reversed if external e.m.f. just greater than the e.m.f. of this cell is applied. Thus in this cell, the net cell reaction can be reversed by applying external opposing e.m.f. greater than the cell e.m.f.
This cell can store electrical energy in form of from external source (charging) and can supply it during discharging. The energy is stored in the form of chemical energy. So this cell is a storage cell or accumulator or storage battery. Its voltage does not depend on the size of the electrodes or the size of its cell but depends upon the strength of a sulphuric acid solution.
Construction:
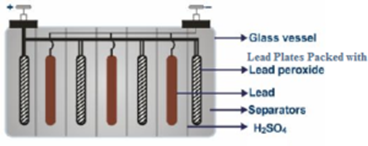
A lead accumulator consists of lead plates as the negative electrode. Lead plates impregnated with lead oxide act as the positive electrode. Negative and positive electrodes are arranged in an alternate manner. This assembly of lead plates is dipped in a non-conducting vessel made up of glass or plastic or ebonite and containing 38% H2SO4 (sp. gr. 1.215). All positive plates are connected to each other and all negative plates are connected to each other.
Representation of Cell:
Pb(s)|PbSO4(s)|38%H2SO4(aq)| PbSO4 (s)| PbO2(s) |Pb(s) +
Working:
Discharging of Cell:
When cell starts working, oxidation takes place at lead plates and reduction takes place at lead plates with lead oxide. This is called as discharging of cell.
2 H2SO4(aq) → 4H+ + 2 SO4 – –
Reaction at negative electrode (Anode)
Pb → Pb++ + 2 e–
Pb++ + SO4– – → PbSO4(s)
Pb(s) + SO4– – → PbSO4(s) + 2e– (oxidation)
Reaction at positive electrode (Cathode)
PbO2(s) + 4H+ + SO4– – + 2e– → PbSO4(s)+ 2H2O(l)
Net cell reaction during discharging
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2 PbSO4(s) + 2H2O(l)
Thus during discharging sulphuric acid is converted into water and hence specific gravity of sulphuric acid solution falls to 1.17.
Charging of cell:
When external emf greater than the emf of this cell is applied, exact reverse reactions take place. At positive electrode oxidation takes place and a negative electrode reduction takes place. This is called the charging of the cell.
Reaction at negative electrode (Cathode)
PbSO4(s) + 2e– → Pb(s) + SO4– –
Reaction at positive electrode (Anode)
PbSO4(s)+ 2H2O(l) → PbO2(s) + 4H+ + SO4– – + 2e–
Net Cell Reaction During Charging
2 PbSO4(s) + 2H2O(l)
Pb(s) + PbO2(s) + 2 H2SO4(aq)
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2 PbSO4(s) + 2H2O(l)
E.M.F. of Cell:
A fully charged accumulator with 38% H2SO4 has a voltage about 2.041 V i.e. approx 2 V.
Uses of Lead Accumulator:
- Lead accumulators are used in motor cars and other automotive vehicles and therefore popularly known as car batteries. Here generally six cells are connected in series to give a voltage of 12 V.
- They are used in laboratories as a source of constant DC voltage.
- They are used in telephone and telegraph offices.
- They are used in electric clocks, radio sets, burglar’s alarms etc.
- They are also used as a source of electricity in the initial stages of the rocket.
Maintenance of Lead Accumulator:
- It is kept erect and should not be tilted.
- When the cell is not in use for a longer time, the terminals should be disconnected and acid from the cell should be completely removed and it is to be kept dry.
- The concentration of sulphuric acid should always be maintained to get the optimum value of cell potential.
- The cell should not be allowed to run down much. When in use the e.m.f. of the cell decreases. When the voltage is about 1.8 V, the cell should be recharged.
- The cell should not be overcharged i.e. e.m.f. of the cell should not be more than 2.1 V.
- The acid level should be maintained properly by adding distilled water.
- Positive and negative terminals of the lead accumulator should be cleaned to remove insoluble lead sulphate from time to time for good electrical contact.
- The cell should be charged regularly and never left in the discharged condition.
Nickel Cadmium Cells:
The first Ni–Cd battery was created by Waldemar Jungner of Sweden in 1899. Nickel-Cadmium battery is a rechargeable battery with Nickel Oxide-Hydroxide (cathode) and Cadmium as a cathode and Alkaline Potassium Hydroxide as the electrolyte.
Reaction at the anode (Oxidation):
Cd(s) + 2OH– (aq) → Cd(OH)2(s) + 2e–
Reaction at the cathode (Reduction):
NiO2(s)+2H2O(l) + 2e– → Ni(OH)2(s) + 2OH– (aq)
Net Reaction:
Cd(s) + 2H2O(l) + 2e– → Cd(OH)2(s) + Ni(OH)2(s)
The reaction products are solid which gets adhered to the respective electrode surface, hence these cells can be recharged. The e.m.f obtained is 1.4 V. These cells have much more life than other dry cells. They are used in electronic watches, calculators and photographic equipment. They have low internal resistance, Can tolerate deep discharge cycle and can also get charged rapidly. These batteries also have a long lifetime and low maintenance costs.
Nickle-Cadmium batteries are costlier than lead-acid battery also the Cadmium used in the battery causes severe environmental pollution.
Previous Topic: Primary Electrochemical Cells
Next Topic: Representation of Electrochemical Cells