Science > Chemistry > Electrochemistry > Types of Cells
There are two types of cells: a) Electrolytic cell and b) Electrochemical cells
Conversion of Chemical Energy into Electrical Energy:
Certain chemical reactions have the capacity to take place spontaneously and can produce electrical energy when carried under appropriate conditions, like proper device, the proper concentration, pressure, and temperature, etc. Chemical energy is converted into electrical energy. At the expense of spontaneous redox (Oxidation-Reduction) reaction, electrical energy is generated.
An electrochemical cell is a device which is used to convert chemical energy into electrical energy. Electrochemical cells are also called as voltaic or galvanic cells. e.g. Daniel cell, Leclanche cell, Dry Cell, lead accumulator etc.
Conversion of Electrical Energy into Chemical Energy:
Certain chemical reactions which have no capacity to take place can be made to take place by supplying an appropriate quantity of electrical energy using a suitable device. In such a change electrical energy is converted into chemical energy. A non-spontaneous redox reaction is made to take place at the expense of electrical energy.
An electrolytic cell is a device which is used to convert electrical energy into chemical energy. In an electrolytic cell, a non-spontaneous redox reaction is made to take place by supplying an appropriate quantity of electrical energy and this process is called electrolysis. e.g. Voltameter used for carrying out electrolysis of acidified water, A device used for electro-refining or electroplating processes.
Cell and its Types:
A cell is a device which is used to convert either electrical energy into chemical energy or chemical energy into electrical energy. Cells are of two types, a) Electrolytic cell b) Voltaic or Galvanic cell
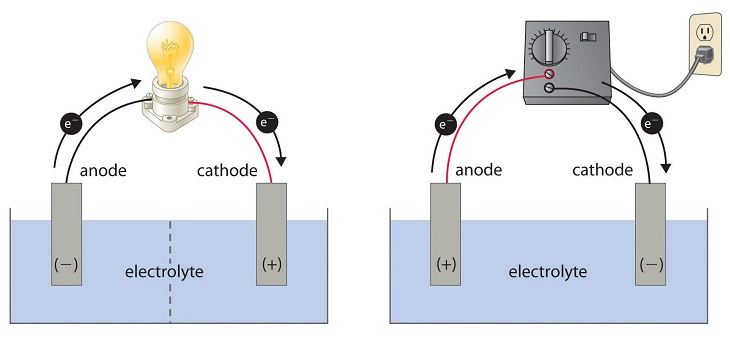
Electrolytic Cell:
- An electrolytic cell is a device which is used to convert electrical energy into chemical energy.
- In an electrolytic cell, a non-spontaneous redox reaction is made to take place by supplying an appropriate quantity of electrical energy and this process is called electrolysis.
- In this cell, electrical energy is used.
- In this cell anode is positive and the cathode is negative.
e.g. Voltameter used for carrying out electrolysis of acidified water, A device used for electrorefining or electroplating processes, charging of storage battery ( Lead accumulator cell)
Voltaic or Galvanic Cell:
- An electrochemical cell is a device which is used to convert chemical energy into electrical energy.
- In electrochemical cell a spontaneous reaction takes place.
- In this cell, electrical energy is produced.
- In this cell anode is negative and the cathode is positive.
e.g. Daniel cell, Leclanche cell, Dry Cell, lead accumulator, etc.
Electrochemical cells are also called as voltaic or galvanic cells.
Important Terms (Terminology):
- Electrolytic Cell: An electrolytic cell is a device which is used to convert electrical energy into chemical energy.
- Electrochemical Cell: An electrochemical cell is a device which is used to convert chemical energy into electrical energy.
- 1 Coulomb (Q): It is the unit of the quantity of electricity. One coulomb is defined as the quantity of electricity that passes through a circuit when a current of one-ampere strength is passed through the circuit for one second.
- 1 Ampere (I): It is the unit of the strength of the electric current. One ampere is defined as the strength of current obtained when one coulomb of electricity is passed through the point electric circuit for one second.
- 1 Volt (V): It is the unit of potential difference. It measures the electromotive force or the electrical pressure driving the electrons through a circuit. One volt is defined as the difference in potential required to pass a current of 1 ampere through 1-ohm resistance.
- 1 Joule (J): It is a unit of both work and energy. One joule is the work done per second by a current of one ampere flowing through a resistance of one ohm. OR One joule is the amount of work performed by a current of one ampere flowing for one second under a potential difference of one volt.
- 1 Watt (W): The electrical power is measured in watt. One Watt is the power in a circuit in which a current of one ampere flows across a potential difference of one volt.
- 1 Faraday (F): It is also the unit of quantity of electricity. One Faraday is the quantity of electricity which is required to deposit or to liberate one gram equivalent (or 10-3 kg equivalent) of any substance from its solution during electrolysis. 1 faraday = 96500 coulombs.
- 1 ohm (Ω): It is the unit of resistance of the circuit to the electric current. One ohm is defined as the resistance between two points in the conductor through which a potential difference of 1 volt will cause a current of one ampere.
- Electrode: Electrodes are metallic or non-metallic rods immersed in the electrolyte. They conduct electric current through them. Carbon and platinum are mainly used electrodes because they are inert and do not get dissolved in the electrolytic solution.
- Cathode: The electrode through which electrons leave the solution or the electrode at which reduction takes place is called the cathode.
- Anode: The electrode through which electrons enter the solution or the electrode at which oxidation takes place is called the anode.
- Cations: Positive ions in the solution take up electrons from the cathode and get discharged. Thus positive ions are getting discharged at the cathode, hence they are called cations. e.g. Na+ is a cation.
- Anions: Negative ions in the solution give up electrons to anode and get discharged. Thus negative ions are getting discharged at the anode, hence they are called anions. e.g. Cl– is an anion.
Electrolyte:
An electrolyte is a substance, which in aqueous solution or in the fused state, liberates ions and allows the electric current to pass through it resulting in the chemical decomposition of the substance. All acids, bases, and salts are electrolytes. e.g. NaCl, KCl, etc.
Non-Electrolyte:
Non-electrolyte is a substance, which in aqueous solution or in the fused state, does not liberate ions and does not allow the electric current to pass through it. e.g. Sugar, urea, etc.
Previous Topic: Ionic Conduction
Next Topic: Electrolysis and its Applications