Science > Chemistry > Electrochemistry > Ionic Conduction
The conductance of an ion depends on its size in an aqueous medium or in the solvent. Bigger is the ionic size lesser is its conductance
Example: The order of size of hydrated ionic radii of alkali metal cations is as Li+(aq) < Na+(aq) < K+(aq)< Rb+(aq)< Cs+(aq). Hence the ease of ionic conductance is Li+(aq) > Na+(aq) > K+(aq) > Rb+(aq) > Cs+(aq)
Concept of Molar Conductivity of an Electrolyte (Λ):
The different solutions may have different concentrations and hence contain a different number of ions. Hence electrolytic conductivity is not a suitable quantity to compare conductance of different solutions. In 1880 the German physicist George Kohlrausch introduced the concept of molar conductivity which is used to compare conductance of different solutions.
The molar conductivity of an electrolyte is defined as the electrolytic conductivity divided by the molar concentration C of the dissolved electrolyte.
Λ = κ / C or Λ = κV
S.I. unit of electrolytic conductivity is siemens per metre (Sm-1) or S cm-1. S.I. unit of molar conductivity is siemens square metre per mole (S m2 mol-1). or S cm2 mol-1
If concentration C is measured in M i.e. mol L-1 or mol dm-3, then the relationship can be written as
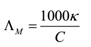
If normality of solution is given then the conductivity is called equivalent conductivity and the relation can be written as
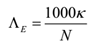
The relation between molar conductivity and equivalent conductivity is
Λ M = n ΛE
Where n is total positive or negative valencies.
Variation of Electrolytic Conductivity with Concentration:
The electrolytic conductivity depends on the number of ions present in a unit volume of a solution. on dilution the degree of dissociation increases. Thus the number of current-carrying ions in the solution increases. But actually, the number of current-carrying ions per unit volume decreases. Hence the activity of the number of ions decreases and hence the electrolytic conductivity also decreases.
For the strong electrolyte, the electrolytic conductivity increases sharply with increasing concentration. For the weak electrolyte, the electrolytic conductivity is very low in dilute solutions and increases much more gradually with increase in the concentration. and this increase is due to an increase in active ions in the solution.
Variation of Molar Conductivity with Concentration:
The molar conductivity of both strong and weak electrolytes increases with dilution i.e. decrease in the concentration.
The molar conductivity is the conductance of all the ions produced by one mole of the electrolyte. Due to an increase in dilution degree of dissociation increases and which results in an increase in the molar conductivity.
For the strong electrolyte, the molar conductivity increases sharply with increasing concentration. Similarly weak electrolyte the molar conductivity increases gradually with an increase in the concentration.

Friedrich Kohlrausch Relation:
Friedrich Kohlrausch performed repeated experiments and plotted a graph of molar conductivity versus the square root of the concentration of a solution.

They showed that the molar conductivity of strong electrolytes varies linearly with the square root of concentration and established the following relation

Where Λ = Molar conductivity at
given concentration
Λo = Molar conductivity at zero concentration or infinite
dilution
C = Concentration of solution
α = constant.
The graph of molar conductivity versus the square root of the concentration of a solution is linear for a strong electrolyte. But such a graph for weak electrolytes is not a straight line.
Kohlrausch Law:
The law states that at infinite dilution, each ion migrates independently of its co-ion and makes its own contribution to the total molar-conductivity of an electrolyte. irrespective of the nature of the other ion with which it is associated.
Thus according to the law at infinite dilution, the total molar conductivity is the algebraic sum of molar conductivities of cation and anion.

Where, Λ = Molar conductivity of a solution
λ +o = Molar conductivity of a cation
λ –o = Molar conductivity of an anion
For electrolyte AmBn, the molar conductivity at infinite dilution is

Illustration:


In both the cases the difference in of K and Na salt is the difference between Λo values of K and Na ions, and it is constant. This illustrates the law.
Applications of Kohlrausch Law:
- The law can be used to calculate the molar-conductivity of any electrolyte at zero concentration.
- The law is particularly useful in the calculation of Λo of weak electrolyte for which extrapolation method is not useful.
- Using the extrapolation method value of Λo for strong electrolytes is found and using that value of Λo weak electrolyte can be calculated.
Calculation of the Molar Conductivity of any Electrolyte at Zero Concentration:
Let us calculate Λo for weak electrolyte acetic acid (CH3COOH|) using Λo values of strong electrolytes sodium acetate (CH3COONa|) and sodium chloride (NaCl).

The values of Λo for strong electrolytes can be found by extrapolation method and using them for weak electrolyte Λo can be calculated.
Relation Between Molar Conductivity and Dissociation Constant (Theory of Weak Electrolyte) :

Where α = degree of dissociation
Λ = Molar conductivity at concentration C
Λo = Molar conductivity at zero concentration
Now, the dissociation constant k for weak electrolyte is given by

This is the relation between dissociation constant and molar conductivity of the weak electrolyte. This relation is called Ostwald’s equation.
Measurement of Conductivity:
The determination of conductivity and molar conductivity of a solution consists of a measurement of the resistance of the solution using Wheatstone’s metre bridge.
The cell used for measurement consists of a glass tube with two platinum plates coated with a thin layer of finely divided platinum called platinum black. The cell is to be dipped in a solution whose resistance is to be measured as shown in fig.
Now conductivity of a cell is given by

The quantity l/a is constant and called cell constant and is defined as the ratio of the distance between the electrodes and the area of cross-section of the electrode. It is denoted by ‘b’
The resistance of the solution is found using Wheatstone’s metre bridge. Using the above relation the conductivity of the solution is calculated. The molar conductivity is obtained by using the formula and value of cell constant b can be obtained using the formula b = kR
The circuit arrangement is as shown below.
Types of Conduction:
Metallic Conduction:
The charge transfer through electronic conductors is called metallic conduction
Characteristics of metallic conduction:
- In this conduction, charge transfer occurs through metal.
- It involves the flow of electrons.
- There is no movement of metal atoms.
- There is no chemical change of metal.
Ionic or Electrolytic Conduction:
The charge transfer through electrolytic conductors is called electrolytic conduction
Characteristics of metallic conduction:
- In this conduction, charge transfer occurs through molten electrolyte or its aqueous solution
- It involves the motion of ions in the solution.
- There is a movement of ions.
- There is a chemical change in an electrolyte.
Previous Topic: Introduction to Electrochemistry
One reply on “Ionic Conduction”
well explained ..thanks