Science > Chemistry > Redox Reactions > Oxidation Number or Oxidation State Oxidation Number OR Oxidation State: The donation of electrons is called the oxidation and the gain of electrons is called the reduction. Oxidation and reduction can further be explained by a knowledge of “Oxidation number”. The oxidation state of an atom in its […]
Tag: Reducing agent
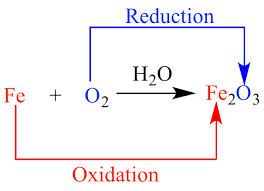
Science > Chemistry > Redox Reactions > Introduction to Redox Reactions In this article we shall study about redox reactions, in which both the oxidation and reduction reactions take place simultaneously. Oxidation: Old Concept: It is a process in which addition of oxygen takes place. 2Mg + O2 → 2MgO It is a […]
There are many observable patterns in the physical and chemical properties of elements as we descend in a group or move across a period in the Periodic Table. The term periodicity is used to indicate that some characteristic properties occur in the periodic table after definite intervals, with a varying (gradual increase or decrease) magnitude. The periodic recurrence of elements having similar […]
Science > Chemistry > Electrochemistry > Electrochemical Series A series of electrodes or half cells arranged in order of their increasing standard oxidation potentials or in the decreasing order of their standard reduction potentials is called an electromotive force series or electrochemical series. Electrochemical series is also known as e.m.f. series Characteristics Electrochemical Series: In […]
Ionic Conduction
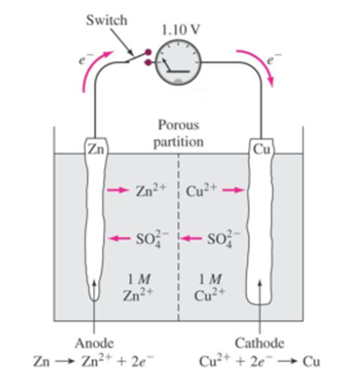
Science > Chemistry > Electrochemistry > Ionic Conduction The conductance of an ion depends on its size in an aqueous medium or in the solvent. Bigger is the ionic size lesser is its conductance Example: The order of size of hydrated ionic radii of alkali metal cations is as Li+(aq) < Na+(aq) < K+(aq)< Rb+(aq)< Cs+(aq). Hence the ease […]

Science > Chemistry > Electrochemistry > Introduction In this article, we shall study the concept of electrochemistry, its cause, and its terminology. Electrochemistry is a branch of chemistry which deals with the interrelationship between chemical energy and electrical energy. The study of electrochemistry is broadly divided into two branches. a) Conversion of chemical energy into […]