Science > Chemistry > Redox Reactions > Introduction to Redox Reactions
In this article we shall study about redox reactions, in which both the oxidation and reduction reactions take place simultaneously.
Oxidation:
Old Concept:
- It is a process in which addition of oxygen takes place.
2Mg + O2 → 2MgO
- It is a process in which addition of electronegative radical takes place.
2FeCl2 + Cl2 → 2FeCl3
- It is a process in which removal of hydrogen takes place.
H2S + 2 [Cl] → S + 2HCl
- It is a process in which removal of electropositive radical takes place.
2KI + H2O2 → I2 + 2KOH
Moden Concept:
According to the electronic concept, a reaction in which loss of electrons from an atom or an ion takes place is called oxidation. Consider reaction,
2Mg + O2 → 2MgO
In this reaction, the valency of magnesium is increased’ from zero (in the atomic state) to + 2 (in MgO).
i.e. Mg0 → Mg2+ + 2 e–
In this reaction, magnesium is losing electrons. And hence oxidation of magnesium takes place.
Reduction:
Old Concept:
- It is a process in which addition of hydrogen takes place.
Cl2 + H2 → 2HCl
- It is a process in which addition of electropositive radical takes place.
2HgCl2 + SnCl2 → Hg2Cl2 + SnCl4 .
- It is a process in which removal of oxygen takes place.
CuO + 2 [H] → Cu + 2H2O
- It is a process in which removal of electronegative radical takes place.
FeCl3 + H → FeCl2 + HCl
Modern Concept:
According to the electronic concept, a reaction in which the gain of electrons by an atom or an ion takes place is called reduction. Consider reaction,
2HgCl2 + SnCl2 → Hg2Cl2 + SnCl4
In this reaction, the valency of mercury is decreased’ from +2 (in HgCl2) to +1 (in Hg2Cl2).
i.e. Hg2+ + e– → Hg +
In this reaction mercury is gaining electron. And hence reduction of mercury takes place.
Redox Reaction:
In any of a chemical reaction if one of the reactants is oxidized, other is surely reduced, i.e. oxidation and reduction always take place simultaneously.
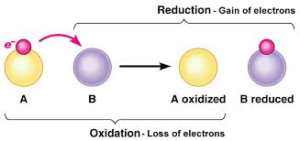
Example – 1:
2Mg + O2 → 2MgO
Mg is oxidized to MgO (addition of oxygen, i.e. increase in positive valency of Mg i.e. loss of electrons by Mg), whereas oxygen is reduced to MgO (addition of positive radical, i.e. increase in negative valency of oxygen, i.e. gain of electrons by oxygen)
Example – 2:
2HgCl2 + SnCl2 → Hg2Cl2 + SnCl4
HgCl2 is reduced to Hg2Cl2 whereas SnCl2 is oxidised to SnCl4.
Thus oxidation and reduction take place simultaneously. Therefore, all such reactions are called as reduction-oxidation reactions or redox reactions. In all such reactions, one of the reactants loses the electrons (oxidized) while other gains those electrons (reduced)
However, it should be remembered that all the chemical reactions are not redox reactions. There are several other types of reactions also.
NaCl + AgNO3 → AgCl + NaNO3
In such reactions none of’ the reactants is oxidized or reduced; simply the exchange of cation or anion takes place.
Oxidizing Agent (Oxidant):
The substance which excepts electrons and makes the other substance to lose electrons is called oxidizing agent or oxidant.
Example – 1:
Consider reaction,
2Mg + O2 → 2MgO
In this reaction, oxygen is making magnesium to lose electrons and hence in this reaction oxygen is the oxidizing agent.
Example – 2:
Consider reaction,
2K + Cl2 → 2KCl
In this reaction, chlorine is making potassium to lose an electron and hence in this reaction chlorine is the oxidizing agent.
Characteristics of Oxidizing Agent:
- The substance which excepts electrons and makes the other substance to lose electrons is called oxidizing agent or oxidant.
- In a reaction, the oxidizing agent oxidizes the other substance but is itself reduced.
- Oxygen, or a substance capable of giving oxygen, is always a good oxidizing agent.
- According to electron concept, an oxidizing agent is that which is capable of de-electronating the other substance.
- An oxidizing agent is an electron acceptor and during the redox reaction, it is electronated.
- Fluorine (F) has a maximum tendency to accept electrons hence it is the strongest oxidizing agent.
Examples of Common oxidizing Agents:
Oxygen (O or O2), Ozone (O3), Hydrogen peroxide (H2O2), Sulphuric acid (H2SO4), Nitric acid (HNO3), Perchloric acid (HClO4), Potassium chlorate (KClO3), Acidified potassium dichromate (K2 Cr2 O7 + H2SO4), Acidified potassium permanganate (KMnO4 + H2SO4), Alkaline potassium permanganate (KMnO4 + KOH)
Reducing Agent (Reductant):
The substance which loses electrons and makes the other substance to gain electrons is called reducing agent or reductant.
Example – 1:
Consider reaction,
2Mg + O2 → 2MgO
In this reaction, magnesium is making oxygen to gain electrons and hence in this reaction magnesium is reducing agent.
Example – 02:
Consider reaction, 2K + Cl2 → 2KCl, In this reaction potassium, is making chlorine to gain an electron and hence in this reaction potassium is reducing agent.
Characteristics of Reducing Agent:
- The substance which loses electrons and makes the other substance to gain electrons is called reducing agent or reductant.
- In a reaction, the reducing agent reduces the other substance but is itself oxidised.
- Hydrogen, or a substance capable of giving hydrogen, is always a good reducing agent.
- According to electron concept, a reducing agent is that which is capable of electronating the other substance.
- A reducing agent is an electron donor and during the redox reaction, it is de-electronated.
- Sodium (Na) has a maximum tendency to donate electron hence it is the strongest reducing agent.
Examples of Common Reducing Agents:
Hydrogen (H or H2), Hydrogen iodide (HI), Hydrogen sulphide (H2S), Lithium aluminium hydride (LiAI H4), Sodium borohydride (NaB H4), Sulphur dioxide (SO2), Carbon (C), Ozone (O3), Hydrogen peroxide (H2O2), Tin & hydrochloric acid (Sn + HCl), Sodium & alcohol (Na + C2 H5OH), Metallic salts (ous) like SnCl2 , FeSO4 etc.
2 replies on “Introduction to Redox Reactions”
Nice
Very nice explanations