Science > Chemistry > Electrochemistry > Introduction
In this article, we shall study the concept of electrochemistry, its cause, and its terminology.
Electrochemistry is a branch of chemistry which deals with the interrelationship between chemical energy and electrical energy. The study of electrochemistry is broadly divided into two branches. a) Conversion of chemical energy into electrical energy and b) Conversion of electrical energy into chemical energy. Electrochemistry has wide applications in engineering and science. Michael Faraday is called the father of electrochemistry.
Basic Terminology of Electrochemistry:
Oxidation Reaction:
The loss of an electron or electrons by a species is called oxidation. Example
Na → Na+ + e–
In oxidation, the oxidation number of elements increases as a result of the loss of electrons. In the above example, the oxidation number of sodium increases from 0 to +1. Thus the oxidation can also be defined as the process in which the oxidation number of an element increase.
Reduction Reaction:
The gain of an electron by a species is called reduction. Example.
Cl + e– → Cl–
In reduction, the oxidation number of an element decreases as a result of the gain of electrons. In the above example the oxidation number of chlorine decreases from 0 to -1. Thus the reduction can also be defined as the process in which the oxidation number of an element decreases.
Oxidizing Agent (Oxidant):
The substance which accepts electrons and makes the other substance to lose electrons is called oxidizing agent or oxidant. Consider reaction
2Mg + O2 → 2MgO
In this reaction oxygen is making magnesium to lose electrons and hence in this reaction oxygen is the oxidizing agent.
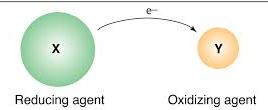
Reducing Agent (Reductant):
The substance which loses electrons and makes the other substance to accept electrons is called a reducing agent or reductant. Consider reaction
2Mg + O2 → 2MgO
In this reaction, magnesium is making oxygen to accept electrons and hence in this reaction magnesium is reducing agent.
Redox Reactions:
In any of a chemical reaction, if one of the reactants is oxidized, the other is surely reduced. Consider reaction
2Mg + O2 → 2MgO
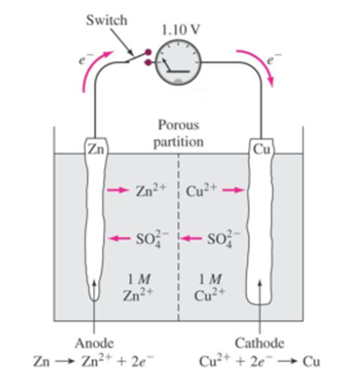
In this reaction, Mg is oxidized to MgO (loss of electrons by Mg), whereas oxygen is reduced to MgO (gain of electrons by oxygen). Hence oxidation and reduction take place simultaneously. Therefore, all such reactions are called as reduction-oxidation reactions or redox reactions. In all such reactions, one of the reactants loses the electrons (oxidized) while other gains those electrons (reduced). Such a reaction may be expressed as the sum of two half-reactions. One reaction involving loss of electrons by a species and another involving gain of electrons by a species. This is the basis of all electrochemical processes.
Conductance:
The substances that allow the flow of electricity through them are called conductors. The flow of electricity through a conductor involves the transfer of electrons from one point to the other. Depending on the mechanism of the transfer of electrons, the conductors are classified into two types. a) Electronic conductors b) Electrolytic conductors
Electronic Conductors:
The conductors through which the conduction of electricity occurs by direct flow of electrons under the influence of applied potential are known as electronic conductors. e.g. copper, aluminium, silver, mercury etc.
Characteristics of Electronic Conductors:
- In electronic conductors, the flow of electricity occurs by the migration of electrons through the conductor.
- In electronic conductors, the conduction does not involve the transfer of matter.
- In electronic conductors, the conduction process does not involve chemical change.
- The resistance of electronic conductors increases and their conductivity decreases with the increase in temperature.
- Ohm’s law is followed but Faraday’s laws are not followed.
Electrolytic Conductors:
The conductors through which the conduction of electricity occurs by the migration of positive and negative ions under the influence of applied potential are known as electrolytic conductors. e.g. electrolysis of fused NaCl.
Characteristics of Electrolytic Conductors:
- In electronic conductors, the flow of electricity occurs by the migration of positive and negative ions through the conductor.
- In electrolytic conductors, the conduction involves the transfer of matter.
- In electrolytic conductors, the conduction process always involves chemical change.
- The resistance of electronic conductors decreases and their conductivity increases with the increase in temperature.
- Both Ohm’s law and Faraday’s laws are followed.
Resistance and Conductance:
By ohm’s law, V = IR
Where R = Resistance of a conductor V = Potential difference across the conductor I = current through the conductor. S. I. unit of resistance is ohm (Ω), that of potential difference is volt (V) and that of current is ampere (A)
Resistance of an Electronic Conducting Wire:
Experimentally it is found that the value of resistance (R) depends on the length (L) of a conductor, the area of cross-section (A) of conductor and nature of a conductor as follows:-
The resistance is directly proportional to the length of a conductor.
R α L ………………. (1)
The resistance is inversely proportional to the area of a cross-section.
R α 1/A ………………. (2)
The resistance depends on the nature of the conductor.
From equation (1) & (2)
R = ρl / A
This is an expression for the specific resistance or the resistivity of a material of a conductor.
Resistivity or Specific Resistance:
We have, ρ = RA / l
Let A = 1 unit and L = 1 unit, then ρ = R
Thus specific resistance or resistivity of a material of a conductor is defined as that resistance of a conductor whose area of cross-section and its length is unity.
Unit of Resistivity or Specific Resistance:
We have, ρ = RA / l
Hence unit of ρ = Unit of R x Unit of Area / Unit of Length = ohm x metre² / metre = ohm metre
Therefore, S.I. unit of resistivity or specific resistance is ohm metre (Ωm)
Conductance:
Reciprocal of resistance is called conductance (K). Its S.I. unit is mho or siemens.
Conductivity:
Reciprocal of resistivity is called as conductivity (κ)
Conductance of an Electronic Conducting Wire:
Experimentally it is found that 1. The conductance (G) is directly proportional to the area of cross-section (A) of a conductor
G α A ………………. (1)
The conductance (G) is inversely proportional to the length (L) of the conductor
G α 1/L ……….. (2)
It also depends on the material of the conductor.
From equation (1) & (2)
G α A / L
G = κA / L
k is constant called specific conductance or conductivity.
This is an expression for the resistance of a conducting wire.
Now, Let A = 1 unit and L = 1 unit , then G = κ
Thus specific conductance or conductivity of a material of a conductor is defined as that conductance of a conductor whose area of cross-section and its length is unity. S.I. unit of conductivity is siemens per metre (S m-1). Other units used are S cm-1, W-1m-1, and W-1cm-1.