Science > Chemistry > Electrochemistry > Electrochemical Series
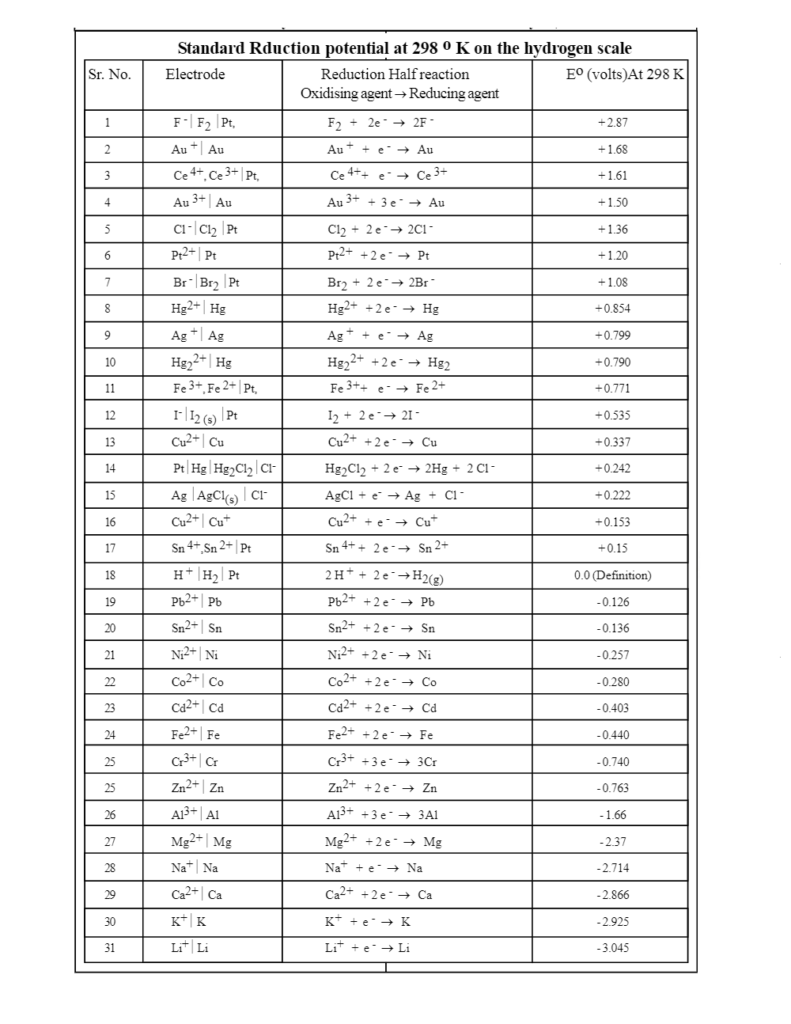
A series of electrodes or half cells arranged in order of their increasing standard oxidation potentials or in the decreasing order of their standard reduction potentials is called an electromotive force series or electrochemical series. Electrochemical series is also known as e.m.f. series
Characteristics Electrochemical Series:
- In this series, all reduction potentials are given on hydrogen scale whose, Eo is taken as zero.
- The standard reduction potential of an element is a measure of the tendency of that element to get reduced.
- The element which has greater reduction potential gets reduced easily. While the elements with low reduction potential will get easily oxidized
- Elements that lose electrons more easily have lower (negative) reduction potential and those which lose electrons with greater difficulty or instead of losing they accept electrons more easily have a higher (positive) reduction potential.
- In EMF series elements having higher (+ ve), the reduction potential is placed at the top. While those having lower (-ve) reduction potential are placed at the bottom. SHE has the middle position in the electrochemical series.
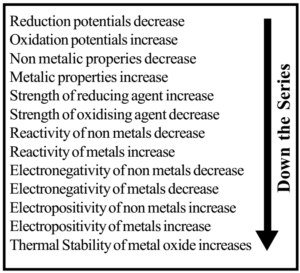
- The substances which are stronger reducing agents than hydrogen are placed below the hydrogen in the series and have negative standard reduction potential. The substances which are weaker reducing agents than hydrogen are placed above the hydrogen in the series and have positive standard reduction potential. Thus as we move down the group strength of reducing agent increases while the strength of the oxidizing agent decreases.
- Metal at the bottom is the most active metal. As we move down in the series activity and electropositivity of metals increase. Nonmetal at the Top is the most active nonmetal. As we move down in the series activity and electronegativity of nonmetal decreases.
Applications of Electrochemical Series:
For Choosing Elements as Oxidising Agents:
The elements which have more electron-accepting tendency are oxidizing agents. Elements at the top of the electrochemical series have higher (+ ve) reduction potential. Hence they gain an electron from other elements and oxidize them. So they are good oxidizing agents.
Element (F2) at the topmost position of electrochemical series which has the highest reduction potential is the strongest oxidizing agent. Oxidizing power decreases from top to bottom in the series.
e.g. The elements like Cu, Ag, Hg, Br2, Cl2, etc. are good oxidizing agents. F2 is the strongest oxidizing agent.
For Choosing Elements as Reducing Agents:
The elements which have more electron losing tendency are reducing agents. The elements at the bottom in the electrochemical series have lower (- ve) reduction potential. Hence they lose electrons readily and supply to other elements and reduce them. So bottom elements in electrochemical series are reducing agents. Reducing strength goes on increasing from top to bottom in the series.
Element (Li) having the bottom-most position has the lowest reduction potential hence it is the strongest reducing agent.
e.g. The element like Zn, Cd, Ni, K, etc. are good reducing agents.
For Studying displacement reaction:
One metal can be displaced from a salt solution by another metal is known as a redox reaction. Elements having higher reduction potential will gain electrons and that having lower reduction potential will lose electrons. Hence element lower in electrochemical series can displace an element placed higher in electrochemical series from its salt solution.
Example-1:
Zn displaces Cu from CuSO4, because, zinc is placed lower in electrochemical series and has lower reduction potential while Cu is placed higher in electrochemical series and has higher reduction potential. Hence zinc can easily displace copper from CuSO4
Zn + CuSO4 → ZnSO4 + Cu i.e.
Zn + Cu++(aq) → Zn ++(aq) + Cu
Example-2:
Fe displaces Cu from CuSO4 because Fe is placed lower in electrochemical series and has lower reduction potential while Cu is placed higher in electrochemical series and has higher reduction potential. Hence Fe can easily displace copper from CuSO4.
Fe + CuSO4 → FeSO4(aq)+ Cu i.e.
Fe + Cu++(aq) → Fe++(aq) + Cu
To predict whether a given metal will displace another, from its salt solution:
A metal lower in the series will displace the metal from its solution which is higher in the series, i.e., the metal having low standard reduction potential will displace the metal from its salt’s solution which has a higher value of standard reduction potential. A metal lower in the series has a greater tendency to provide electrons to the cations of the metal to be precipitated.
Displacement of one nonmetal from its salt solution by another nonmetal:
A nonmetal higher in the series having the high value of standard reduction potential will displace another nonmetal with lower reduction potential i.e., occupying the position below in the series. The nonmetal’s which possess high positive reduction potentials have the tendency to accept electrons readily. These electrons are provided by the ions of the nonmetal having the low value of reduction potential. Thus, Cl2 can displace bromine and iodine from bromides and iodides.
Displacement of hydrogen from dilute acids by metals:
The metal which can provide electrons to H+ ions present in dilute acids for reduction evolve hydrogen from dilute acids. The metal having negative values of reduction potential possess the property of losing electron or electrons. Thus, the metals occupying lower positions in the electrochemical series readily liberate hydrogen from dilute acids and on ascending in the series tendency to liberate hydrogen gas from dilute acids decreases.
The metals which are above hydrogen in electrochemical series like Cu, Hg, Au, Pt, etc., do not evolve hydrogen from dilute acids.
Displacement of hydrogen from water:
Iron and the metals below iron are capable of liberating hydrogen from water. The tendency increases from top to bottom in electrochemical series. Alkali and alkaline earth metals liberate hydrogen from cold water but Mg, Zn and Fe liberate hydrogen from hot water or steam.
For Calculation of standard EMF of cell ( Eocell):
From the standard electrode potential values, it is easy to calculate EMF of cell. Standard oxidation potential values are given in EMF series. Eo cell is calculated using formula:
Eocell = Eored (cathode) – Eored (anode)
e.g. in Daniell cell,

Now, From the series, EoZn = – 0.763 V , EoCu = + 0.337 V
Eocell = Eored (cathode) – Eored (anode)
∴ Eocell = Eored (Cu) – Eored (Zn)
∴ Eocell = 0.337 – ( -0.763)
∴ Eocell = 0.337 + 0.763
∴ Eocell = 1.10 V
For Checking Spontaneity of Redox Reactions:
If cell is assembled such that one electrode has higher positive oxidation potential and other has lower negative oxidation potential then redox cell reaction will be spontaneous and cell will have positive EMF. On the contrary if EMF of cell is negative then redox cell reaction will be non spontaneous.
e.g. in Daniell cell,

Now, From the series, EoZn = – 0.763 V , EoCu = + 0.337 V
Eocell = Eored (cathode) – Eored (anode)
∴ Eocell = Eored (Cu) – Eored (Zn)
∴ Eocell = 0.337 – ( -0.763)
∴ Eocell = 0.337 + 0.763
∴ Eocell = 1.10 V
Since cell has positive EMF, following redox cell reaction is spontaneous.
Zn + Cu++(aq) → Zn ++(aq) + Cu
Thus higher the positive EMF of the cell, the more is the spontaneity of the redox cell reaction.
For Construction of a Cell:
Various cells can be constructed by combining standard electrodes given in EMF series as per the requirement of e.m.f.
e.g If a cell of e.m.f. 1.1 V is required, then from e.m.f. series we can locate zinc and copper electrode whose combination gives required e.m.f.
For Corrosion Treatment:
When two metals which are in contact with each other are exposed to the atmosphere, the element lower in series will be oxidized. i.e. it is rusted and destroyed.
If there is a scratch on the galvanized sheet of iron, and iron is exposed then zinc is rusted and iron is protected. This is because in e.m.f. series zinc is below the iron. But if there is a scratch on the tin-plated iron, iron gets rusted because in e.m.f. series iron is below tin.
To Find Reactivity of Metals:
As we move down in the electrochemical series reactivity of metal increases. Alkali metals and alkaline metals at the bottom are highly reactive. They can react with cold water and evolve hydrogen. They can dissolve in acid-forming salt.
Metals like Fe, Pb, Sn, Ni, Co which are in little higher in the series do not react with cold water but react with steam and evolve hydrogen. Metals like Cu, Ag, and Au which lie above the hydrogen are less reactive and do not react with water in any form to evolve hydrogen.
To Ascertain Electropositivity of Metals:
Strongly electropositive metals:
Metals having standard reduction potential near about -2.0 volt or more negative like alkali metals, alkaline earth metals are strongly electropositive in nature.
Moderately electropositive metals:
Metals having values of standard reduction potentials between 0.0 and about -2.0 volt are moderately electropositive. Al, Zn, Fe, Ni, Co, etc., belong to this group.
Weakly electropositive metals:
The metals which are above hydrogen and possess positive values of standard reduction potentials are weakly electropositive metals. Cu, Hg, Ag, etc., belong to this group.
To Find Thermal Stability of Metallic Oxides:
The thermal stability of the metal oxide depends on its electropositive nature. As the electropositivity increases from top to bottom, the thermal stability of the oxide also increases from top to bottom.
The oxides of metals having high positive reduction potentials are not stable towards heat. The metals which are above copper form unstable oxides, i.e., these are decomposed on heating.
To Determine the Products of Electrolysis:
In case two or more types of positive and negative ions are present in solution, during electrolysis certain ions are discharged or liberated at the electrodes in preference to others.
In general, in such competition, the ion which is the stronger oxidizing agent (higher value of standard reduction potential) is discharged first at the cathode. e.g. In a mixture of copper and silver ions, silver will be deposited first because the reduction potential of silver is higher than copper.
- The increasing order of deposition of few cations is: K+, Ca++, Na+, Mg+++, Al+++, Zn++, Fe++, H+, Cu++, Ag+, Au+++.
- The anion which is a stronger reducing agent (low value of standard reduction potential) is liberated first at the anode.
- The increasing order of discharge of few anions is SO4– –, NO3–, OH–, Cl–, Br–, I–
- When an aqueous solution of NaCl containing Na+, Cl–, H+, and OH- ions is electrolyzed, H+ ions are discharged at cathode and Cl- ions at the anode, i.e., H2 is liberated at cathode and Cl2 at the anode.
- When an aqueous solution of CuS04 containing Cu++, H+ and OH- ions is electrolyzed, Cu++ ions are discharged at the cathode and OH– ions at the anode.
Previous Topic: Use of Nernst Equation
13 replies on “Electrochemical Series and its Applications”
Thank you.
Thanks very cleared.
Very great and interesting
Thanks for the clarification
Thanks its very useful. Clear explanation
Thanks seen and cleared explanation
Thank you, it is really helpful.
Ohh wonderfull explanation
well explained
Holy crap!
This was very very very helpful… Thank you 🥺
GOD BLESS
I have understand it all
Thank God bless you.
Thanks
Thanks A Lot. Namastey!