Science > Chemistry > Atomic Structure > Electronic Configuration
The distribution of electrons in various shells and orbitals of an atom is known as electronic configuration. Generally electronic configuration is given by nlx. Where n is shell number, l is subshell and x are the number of electrons.
Aufbau Principle:
The arrangement of the electrons in an atom is called the electronic configuration of an atom. The method of filling up or building up a sequence of energy levels for electrons in an atom is based on Aufbau principle. In German, the word aufbau means building up.
According to this principle, orbitals are filled in the order of increasing energy. The lowest energy level is filled first. The higher energy levels are then filled up one after another. This gives the atom the maximum stability and the atom is said to be in ground state or the normal state.
The order of increasing energy levels is 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p < 6f Diagrammatically it is represented as follows
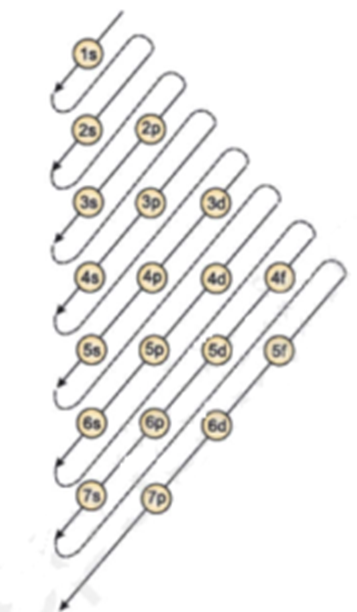
Pauli’s Exclusion Principle:
“No two electrons. in an atom can have the same values for all four quantum numbers”. i.e. to exist in the same atom the two electrons can have three quantum numbers identical but fourth must be different.
Example: Let us consider electrons in the first orbit of helium atom
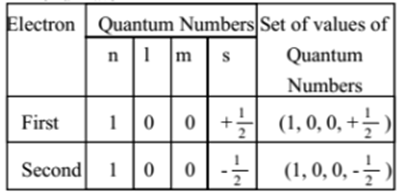
Here we can see that the three values of quantum number are the same but fourth value is different. Thus Pauli’s exclusion principle helps in calculating the maximum number of electrons present in any energy level (Orbit).
Hund’s Rule of Maximum Multiplicity:
In a given subshell, electrons occupy all the available orbitals singly, before they pair up in any orbital.
Example 1:
Consider electronic configuration of phosphorous. The atomic number of phosphorous is 15. Its basic electronic configuration is 2, 8, 5. The detailed electronic configuration is 1s2, 2s2 2p6, 3s2 3p3. The box diagram for final orbit configuration for P is as below

Here we can see that the electrons in p orbitals occupy the position singly till all orbitals are singly filled.
Example – 2:
Consider electronic configuration of sulphur. The atomic number of sulphur is 16. Its basic electronic configuration is 2, 8, 6. The detailed electronic configuration is 1s2, 2s2 2p6, 3s2 3p4. The box diagram for final orbit configuration for S is as below

Here we can see that the electrons in p orbital occupy the position singly till all orbitals are singly filled and then the pairing starts.
Stability of Completely Filled and Half Filled Subshells:
The ground state electronic configuration of the atom of an element always corresponds to the state of the lowest total electronic energy.
The electronic configurations of most of the atoms follow the basic rules viz. Aufbau principle, Pauli’s exclusion principle, and Hund’s rule. However, in certain elements such as Cu, or Cr, where the two subshells (4s and 3d) differ slightly in their energies, an electron shifts from a subshell of lower energy (4s) to a subshell of higher energy (3d), provided such a shift results in all orbitals of the subshell of higher energy getting either completely filled or exactly half filled.
The valence electronic configurations of Cr and Cu, therefore, are 3d5 4s1 and 3d10 4s1 respectively and not 3d4 4s2 and 3d9 4s2. It has been found that there is extra stability associated with these electronic configurations.
Electronic Configuration of copper (Z = 29):
Expected configuration: 1s2, 2s2 2p6, 3s2 3p6, 4s2 3d9.
Actual configuration: 1s2, 2s2 2p6, 3s2 3p6, 4s1 3d10.
Electronic Configuration of chromium (Z = 24):
Expected configuration: 1s2, 2s2 2p6, 3s2 3p6, 4s2 3d4.
Actual configuration: 1s2, 2s2 2p6, 3s2 3p6, 4s1 3d5.