Science > Chemistry > Surface Chemistry > Factors Affecting Adsorption
In last article, we have studied the meaning of terms like adsorption, absorption, adsorbate, adsorbent. In this article, we shall study the factors affecting adsorption.
The Extent of Adsorption:
The quantity of an adsorbate that is adsorbed per unit mass of adsorbent is called extent of adsorption.
Thus, a = x/m
Where a = Extent of adsorption
x = The mass of gas adsorbed
m = Mass of adsorbent
The Factors Affecting Adsorption:
Temperature:
Adsorption is an exothermic process, Hence according to Le Chatelier’s principle at given pressure low temperature favours adsorption. If the temperature is increased, adsorbate molecules get removed from the adsorbent and this process is called as desorption.
Thus, adsorption is inversely proportional to the temperature. This is true for physical adsorption. In chemical adsorption due to the high energy of activation, the extent of adsorption increases initially and decreases as the temperature increases further.
Pressure:
When a gas is adsorbed on the surface of adsorbent there is a decrease in the volume of adsorbent. Thus, by Le Chatelier’s principle If the temperature is kept constant, the quantity of gas adsorbed by metal adsorbent increases with the increase in pressure. Adsorption is directly proportional to the pressure of a gas over a limited range of pressure.
Graphical representation:
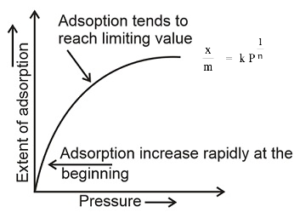
At low pressure:
at low pressure 1/ n = 1
∴ x/m ∝ P
Thus at low pressure, the extent of adsorption is directly proportional to the pressure and hence for low pressure, the graph is a straight line.
At medium pressure:
At medium pressure 0 < 1/ n < 1
∴ x/m ∝ P1/n
Thus at medium pressure, the extent of adsorption increases less rapidly with increase in the pressure hence for medium pressure the graph is a curve.
At high pressure :
At high pressure 1/ n = 0
∴ x/m = constant
Thus at high pressure, the extent of adsorption is independent of pressure hence for high pressure, the graph is parallel to pressure axis.
Nature of Adsorbent:
Since adsorption is a surface phenomenon, adsorption increases with the increase in the surface area of the adsorbent. More finely divided or rougher the surface of adsorbent, the greater will be the surface area and hence the greater will be the adsorption. Metal catalysts in the finely divided form, colloidal form, rough surfaces, activated adsorbent provides more surface area.
Chemisorption is preferential and more specific. A gas will be chemisorbed on a given solid only if it can provide large surface area. For e.g. Hydrogen gas is adsorbed by nickel but not by iron. The chemical nature of the adsorbent should be such so that it can cause chemisorption of adsorbate on the adsorbent.
Nature of Adsorbate:
In case of adsorption of gases by solids, it has been found that more easily liquefiable and highly water-soluble gases are adsorbed more readily due to greater van der Walls forces. Hence ammonia, hydrogen chloride, chlorine and sulphur dioxide are more adsorbed than hydrogen, nitrogen, oxygen.
In physical adsorption, the extent of adsorption depends on the boiling point of the gases. Gases are adsorbed on solids more readily than liquids.
Concentration of Adsorbate:
When liquid is adsorbed on solid, at higher concentration of adsorbate, the extent of adsorption is greater, provides that the temperature is kept constant. The concentration of adsorbate has a similar type of effect as that of pressure.
Adsorption Isotherm:
An adsorption isotherm is a relation between the extent of adsorption (amount of a substance adsorbed per unit mass of an adsorbent) and the equilibrium pressure or concentration at a constant temperature. Actually, it is a curve obtained by plotting extent of adsorption (amount of a substance adsorbed per unit mass of an adsorbent) against the equilibrium pressure or concentration at a constant temperature.
Freundlich Adsorption Isotherm:
Adsorption isotherm is a curve obtained by plotting extent of adsorption (amount of a substance adsorbed per unit mass of an adsorbent) against the equilibrium pressure or concentration at a constant temperature.

An empirical equation for the variation of gas adsorption with pressure at a constant temperature over a limited range of pressure which was put forwarded by Freundlich and is known as “Freundlich adsorption isotherm.” Mathematically the equation is,

Where x = mass of adsorbate (gas) adsorbed.
m = mass of adsorbent (solid).
P = equilibrium pressure of adsorbate.
C = equilibrium concentration of adsorbate in the solution.
In this equation ‘K’ and ‘n’ are constant and ‘n’ is less than one.
At low pressure, the extent of adsorption is directly proportional to the pressure and hence for low pressure, the graph is a straight line. At medium pressure, the extent of adsorption increases less rapidly with increase in the pressure hence for medium pressure the graph is a curve. At high pressure, the extent of adsorption is independent of pressure hence for high pressure the graph is parallel to pressure axis. i.e. x/m = constant. Thus Freundlich adsorption isotherm holds good only at moderate pressure.
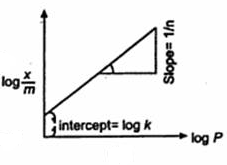
By taking log of both the side of isotherm,

The form of the equation is y = mx + c. On plotting (log x/m) against (log P), a straight line graph is obtained which indicates the validity of the equation. Thus the slope of the graph = 1/n and its y-intercept is log k. From this graph values of k and n can be found out.

2 replies on “Factors Affecting Adsorption”
this article is very clear and simple to understand. rate a 10/10
Very easy understandable and clear