Science > Chemistry > Laws of Chemical Combinations > Law of Conservation of Mass
In last article, we have studied Dalton’s atomic theory. In this article, we shall study the law of conservation of mass. The law of conservation of mass was given by Russian scientist Lomonosov in 1765 and French Scientist Antoine Lavoisier in 1783 independently. This law is also called the law of indestructibility of matter.

Statement:
In a chemical reaction, the total mass of the reactants before the reaction is the same as the total mass of the products after reaction. i.e. in a chemical reaction, the total mass is conserved.
Explanation:
Consider following chemical reaction
A + B → C + D
Let MA = Mass of reactant A
MB = Mass of reactant B
MC = Mass of product C
MD = Mass of product D
By law of conservation of mass
Total mass of reactants = Total mass of products
MA + MB = MC + MD
lllustration:
Consider following chemical reaction
NaCl + AgNO3 → NaNo3 + AgCl ↓
The total mass of reactants = ( 23×1 + 35.5×1) + (108×1 + 14×1 + 16×3)= 228.5
The total mass of products = (23×1 + 14×1 + 16×3) + (108×1 + 35.5) = 228.5
Thus, The total mass of reactants is equal to the total mass of products. Thus, the law of conservation of mass is illustrated.
Landolt’s Experiment:
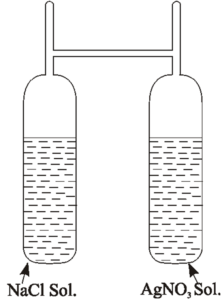
Landolt used this experiment to verify the law of conservation of mass. He used H – Shaped tube for his experiment. One arm (limb) of the apparatus is filled with sodium chloride solution and the other arm is filled with silver nitrate solution.
The tube is then sealed and weighed accurately and carefully so that the two solutions do not mix with each other.
Then the apparatus is well shaken and the two solutions are allowed to mix and react with each other white precipitate of AgCl is obtained.
NaCl + AgNO3 → NaNo3 + AgCl ↓
The apparatus is weighed accurately again. Within experimental limit, it is found that the weight of the apparatus before the reaction is equal to the weight of the apparatus after the reaction. Thus the total mass of reactants is equal to the total mass of the products. Thus the total mass is conserved. Thus the law of conservation of mass is verified.
Limitations of Landolt’s Experiment:
- The reaction between sodium chloride and silver nitrate is exothermic. Due to which evaporation of the moisture on the outer surface of the apparatus takes place and this takes a long time to recondense back on the apparatus.
- There is an increase in the volume of the apparatus, due to the evolution of heat. It takes a long time to get original volume. Due to increase in the volume the up thrust of air on the apparatus increases and hence it shows less weight.
- These errors can be minimized by weighing the apparatus after very long time allowing the apparatus to cool thoroughly.
Limitations of the Law of Conservation of Mass:
The law of conservation fails for chemical reactions in which a large amount of heat is evolved or absorbed during the reaction. During a chemical reaction, energy is evolved or absorbed in the form of heat, light etc. During the evolution of heat, some mass of reactants is converted into energy. But this loss of mass is so small that it cannot be recorded on very sensitive balance.
According to Einstein’s theory, mass and energy are interconvertible. The relation between the two quantities is given by E = mc² Where, E = energy liberated, m = mass lost in the reaction. c = speed of light in vacuum Thus the law of conservation of mass is valid if both the mass and the energy are conserved.
Combined Law of Conservation of Mass and Energy:
In a chemical reaction, the total amount of mass and energy of reactants is equal to the total amount of mass and energy of products i.e. In a chemical reaction the total amount of mass and energy are conserved.
Law of Conservation of Energy: Energy can neither be created nor destroyed, but it can be converted from one form to another. i.e. the total amount of energy of the universe is conserved.
Explanation of the Law of Conservation of Mass on the Basis of Dalton’s Atomic Theory:
According to atomic theory, atoms can neither be created nor be destroyed. In chemical reactions, the atoms rearrange, but they do not themselves break apart. Since each atom has a definite mass, the total mass after chemical change remains constant and this explains the law of conservation of mass.
Numerical Problems:
Example – 01:
10.0 g of CaCO3 on heating gave 4.4 g of CO2 and 5.6 g of CaO. Show that these observations are in agreement with the law of conservation of mass.
Solution:
CaCO3 → CaO + CO2
Mass of reactants = 10.0 g
Mass of products = 5.6 g + 4.4 g = 10.0 g
Thus, Mass of reactants = Mass of products.
Hence the observations are in agreement with the law of conservation of mass.
Example – 02:
x g of potassium chlorate on decomposing produced 1.9 g of oxygen and 2.96 g of potassium chloride. What is the value of x?
Solution:
KClO3 → KCl + O2
Mass of reactants = x g
Mass of products = 4.86 g
Now, by the law of conservation of mass. Mass of reactants = Mass of products.
x = 4.86
The value of x is 4.86
Example – 03:
When 4.2 g of NaHCO3 is added to a solution of CH3COOH weighing 10 g; it is observed that 2.2 g of CO2 is released into the atmosphere. The residue is found to weigh 12.0 g. Show that these observations are in agreement with the law of conservation of mass.
Solution:
NaHCO3 + CH3COOH → Residue + CO2
Mass of reactants = 4.2 g + 10 g = 14.2 g
Mass of products = 12.0 g + 2.2 g = 14.2 g
Thus, Mass of reactants = Mass of products.
Hence the observations are in agreement with the law of conservation of mass. Now, by the law of conservation of mass.
Example – 04:
When 6.3 g of NaHCO3 is added to 15.0 g solution of CH3COOH; the residue is found to weigh 18.0 g. What is the mass of CO2 released in the reaction?
Solution:
NaHCO3 + CH3COOH → Residue + CO2
Mass of reactants = 6.3 g + 15.0 g = 21.3 g
Mass of products = 18.0 g + Mass of CO2
Now, by the law of conservation of mass. Mass of reactants = Mass of products.
∴ 21.3 g = 18.0 g + Mass of CO2
∴ Mass of CO2 = 21.3 g – 18.0 g = 3.3 g
Ans: The mass of CO2 released is 3.3 g.
In the next article, we shall study the law of definite/constant/fixed proportions
Previous Topic: Dalton’s Atomic Theory
Next Topic: The Law of Definite Proportions
One reply on “Law of Conservation of Mass”
thankyou very much