Science > Chemistry > Electrochemistry > Nernst Theory
In this article, we shall study the Nernst theory of electrode potential, Nernst equation, and its use.
Single Electrode or Half cell or Electrode Couple:
A single electrode or half cell or electrode couple is produced when a metal is dipped in the solution of its own ions.
e.g. Cu | Cu++(aq), Zn| Zn++(aq) etc
A single vertical line indicates physical contact between the metal and its ions. Sometimes a couple is produced from gas and solution of its ions. In such cases noble metal like platinum is used as a conductor to adsorb the gas.
e.g. Pt| H2(g) | H+(aq)
Concept of electrode potential (Nernst Theory):
Nernst in 1889 gave his theory of electrode potential. An electrode is a couple of active element, and its ionic solution. When metal is immersed in its salt solution it shows two opposite tendencies called de-electronation (oxidation) and electronation (reduction).
Metals have a tendency to pass into solution as cations and liberate electrons. This process is oxidation or de – electronation. The tendency of a metal to pass into its salt solution in the form of cations liberating electrons is called the solution pressure of metal (Ps). There is a reverse tendency of cations to deposit on the electrode by taking electrons. This process is called as electronation or reduction. The tendency of the ions in the solution to be deposited back on the surface of the metal by taking electrons is called an osmotic pressure of ions (Po).
Nernst suggested the mechanism of the establishment of the difference of potential in a cell. His theory is based on the theory of electrolytic dissociation and his ideas of solution pressure and formation of the electrical double layer.
De-electronation:
The process in which an atom or ion of an element loses one or more electrons is called de-electronation. De-electronation takes place at the electrode when the solution pressure of metal is greater than its osmotic pressure.
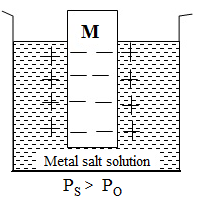
Electrons released in oxidation are accumulated on the electrode and solution, on the other hand, acquires excess positive charge because of the excess of cations. This gives rise to the electrical double layer at the electrode surface. Because of this electrical double layer, a potential difference is set up. As this potential is due to the oxidation, it is called oxidation potential.
e.g. In Daniel cell solution pressure of zinc is more. Zinc passes into its ion solution as Zn ++ ions and electrons released in oxidation get accumulated on the zinc rod. Thus de-electronation takes place at zinc half cell in a Daniel cell.
Zn(s) → Zn++(aq) + 2e–
Thus at zinc electrode, a negative potential is developed which is due to the oxidation.
Electronation:
The process in which an atom or ion of an element gains one or more electrons is called electronation. Electronation takes place at the electrode when the osmotic pressure of metal is greater.

Due to electronation the electrode loses electrons continuously and acquires a positive charge. And the solution, on the other hand, acquires an excess negative charge. Thus electrical double layer is set up across the surface of the metal. Because of the electrical double layer potential difference is set up. As this potential is due to reduction it is called a reduction potential.
e.g. In Daniel cell osmotic pressure of copper ions is more. Cu++ ions from solution take electrons and deposited on the surface of the metal. Thus due to reduction, there is a removal of electrons from the metal surface. Thus electronation takes place at the copper half-cell in Daniel cell.
Cu++(aq) + 2e– → Cu(s)
Thus at the copper electrode, a positive potential is developed due to reduction.
Rate of Electronation or De-electronation:
The rate of de-electronation and electronation differs from metal to metal. There are three possibilities.
Ps > Po: When the solution pressure is greater than the osmotic pressure. The rate of de-electronation is greater than the rate of electronation. The electrode undergoes oxidation. Thus negative potential develops on the electrode and it acts as an anode.
Po > Ps: When the osmotic pressure is greater than the solution pressure. The rate of electronation is greater than the rate of de-electronation. The electrode undergoes a reduction. Thus positive potential develops on the electrode and it acts as a cathode.
Ps = Po: When the solution pressure is equal to the osmotic pressure. The rate of electronation is equal to the rate of de-electronation. Thus there is no double layer formation and hence no potential is developed on the electrode. Such an electrode is called the null electrode.
Nernst Equation:
For single electrode potential: Let M be the metal, ‘n’ be the number of electrons involved in the electrode. Then the reactions are,
M → Mn+ + n e– (oxidation) OR
Mn+ + n e– → M (reduction)
According to the Nernst equation at 25° C
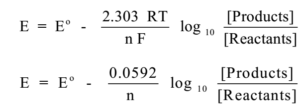
Where, Eo = standard oxidation potential
E = oxidation potential
R = 8.314 J K–1 mol –1
n = no. of electrons involved in electrode reaction.
F = Faraday’s constant = 96500 C
T = temperature in K
[Oxidation state] = concentration of Mn+ ions in mol dm-3
[Reduced state] = activity of pure metal = 1
This expression gives variation of electrode potential with respect to electrolyte concentration.
The first part of the equation represents standard state electrochemical conditions and the second term is a correction for non-standard state electrochemical conditions.
Mathematical expression for EMF of a cell:
Let us consider general cell reaction
aA + bB → cC + dD
Let ‘n’ be the number of electrons in cell reaction.
Then according to Nernst equation, EMF of cell is given by

Calculation of Cell Potential Using Nernst Equation:
Consider a cell
Zn(s)| Zn++(aq)|| H+(aq) (1 M)|H2(g) (1 atm.) | Pt +
Oxidation reaction at anode:
Zn(s) → Zn++(aq) + 2e–
Reduction reaction at anode:
2H+(aq) + 2e– → H2(g)
Net cell reaction:
Zn(s) + 2H+(aq) → Zn++(aq) + H2(g)
The e.m.f. of a cell at 25 °C by Nernst equation is given by

Where concentrations are in mol dm-3 and pressure is in atm.
Calculation of Electrode Potential Using Nernst Equation:
Consider an electrode Zn(s)| Zn++(aq)
Reduction reaction for it is
Zn(s) → Zn++(aq) + 2e–
The electrode potential at 25 °C by Nernst equation is given by

at 25 °C. Where concentrations are in mol dm-3 and pressure is in atm.
Previous Topic: Types of electrodes
Next Topic: Use of Nernst Equation