Science > Chemistry > Solid State > Semiconductors
On the basis of electrical conductivity, substances can be classified into three types conductors, insulators, and semiconductors. In this article, we shall have a brief idea of semiconductors.
Semiconductors are the substances whose conductivity lies between the conductors and insulators e.g. Germanium, Silicon, etc. They are covalent solids. These elements are members of the fourth group of the periodic table with outer orbit configuration ns2 np2 and valency 4. There are no free electrons for conduction in semiconductors at low temperature (absolute zero). Thus germanium crystal acts as an insulator at absolute zero. As the temperature increases, the energy gap reduces and some electrons jump to the conduction band. Thus the conductivity of semiconductors increases with the increase in the temperature. Such semiconductors are also called as extrinsic or pure semiconductors. The conductivity of semiconductors can be increased by purposely adding pentavalent or trivalent impurity (doping) to their crystal in small traces.
The structure of Pure Silicon (Semiconductor) Crystal:
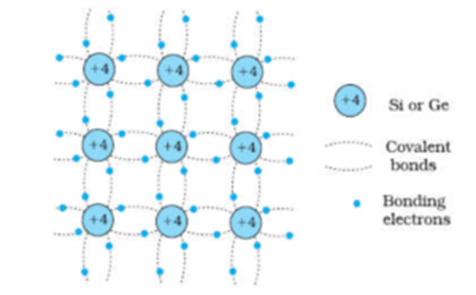
We can see that each silicon atom share its four valence electrons with neighbouring 4 silicon atoms to form four covalent bonds. Thus at absolute zero, all the electrons are localized and not available for conduction. Thus at absolute zero silicon behaves as an insulator.
Types of Semiconductors:
Depending upon the working semiconductors are classified into two types.
Intrinsic semiconductors:
A semiconductor which is in extremely pure form is called intrinsic semiconductor e.g. Germanium, Silicon. The crystal structure of these elements consists of regular repetition in three dimensions of a unit cell having the form of a tetrahedron, with one atom at each vertex. 3. The two-dimensional representation is as shown below.

Consider a semiconductor like germanium having valency four. Germanium atom has four electrons in its outermost shell. Germanium has a crystalline structure in which each atom of germanium shares its valence electrons with four neighboring atoms forming four covalent bonds. The covalent bonds are strong bonds. Thus there is no free electron for conduction in germanium at low temperature (absolute zero). Thus germanium crystal acts as an insulator at absolute zero.
At room temperature, the thermal energy of some electrons increases and they are set free. These free electrons get delocalized and are available for conduction. When these electrons get delocalized, a hole is created at their position. Thus the crystal shows a small conductivity.
Extrinsic semiconductors:
The crystal of intrinsic semiconductors shows a small conductivity. The conductivity of semiconductors can be increased by adding a small quantity of some impurity in the pure crystal of the semiconductor. This process is called doping. The ratio of impurity is very low i.e. 1 atom of impurity for every 106 to 1010 atoms of semiconductors. These atoms of impurities are so less that they do not affect the crystal structure of the semiconductor. Generally, trivalent or tetravalent elements are added as impurities to semiconductor crystal. Depending upon the impurity the semiconductors are classified into two types a) p-type semiconductor and b) n-type semiconductor
p-type Semiconductor:
Let us suppose the germanium is doped with an element from the third group say boron (trivalent impurity). Boron has three Valency electrons. Therefore, boron can form only three covalent bonds with neighboring germanium atoms. One of the covalent bonds around each boron atom has an electron missing. The absence of an electron is called a hole. This impurity is called acceptor impurity.
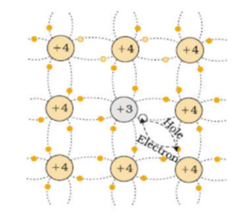
Under the action of an electric field, an electron from a neighboring completely filled covalent bond jumps into this hole creating a hole in the bond from which electron has moved. The process is repeated continuously. Thus the hole appears to move through the crystal from a positive end to a negative end. Thus the conductivity of doped germanium increases. The absence of an electron in the hole means the presence of a positive charge. Hence the doped material is called p-type semiconductor.
Characteristics of p-type Semiconductors:
- In p-type semiconductors, doping is done with trivalent impurity i.e. impurity from the third group of the periodic table.
- The impurity in the p-type semiconductor is called the acceptor impurity.
- Each atom of impurity creates a hole in the crystal.
- The electrical conductivity is due to the hole.
- When a potential difference is applied across the p-type of semiconductor, the holes appear to move from a positive end to a negative end.
- In p-type semiconductors, holes are the major charge carriers.
n-type Semiconductors:
Let us suppose the germanium is doped with an element from the fifth group say phosphorous (pentavalent impurity). Phosphorus has five valence electrons. Therefore, phosphorus can form four covalent bonds leaving one free electron unbonded. Due to pentavalent doping the number of free electrons increases. This impurity is called the donor impurity.

Under the action of an electric field, free-electron around phosphorous moves through the crystal from the negative end to a positive end. Thus the conductivity of doped germanium increases. The presence of an electron means the presence of a negative charge. Hence the doped material is called an n-type semiconductor.
Characteristics of n-type Semiconductors:
- In n-type semiconductors, doping is done with pentavalent impurity i.e. impurity from the fifth group of the periodic table.
- The impurity in the n-type semiconductor is called the donor impurity.
- Each atom of impurity leaves one free electron in the crystal.
- The electrical conductivity is due to electron set free by the electron.
- When a potential difference is applied across n-type of semiconductor, the electrons move from a negative end to a positive end.
- In n-type semiconductors, electrons are the major charge carriers.
Uses of Semiconductors:
- Various combinations of n-type and p-type semiconductors are used for making electronic components.
- A diode is a combination of n-type and p-type semiconductors and is used as a rectifier.
- Transistors are made by sandwiching a layer of one type of semiconductor between two layers of the other type of semiconductor. NPN and PNP types of transistors are used to detect or amplify radio or audio signals.
- The solar cell is an efficient photodiode used for the conversion of light energy into electrical energy. It consists of both p-type and n-type semiconductors.