Science > Chemistry > Solid State > Introduction to Solid State
There are three states of matter, solid, liquid and gaseous. Liquids and gases are called fluids because of their ability to flow. The fluidity in both of these states is due to the fact that the molecules are free to move about. The free mobility of the molecules is due to weak intermolecular forces. In this article series, we shall discuss the solid state of a substance.
The constituent particles in solids have fixed positions and can only oscillate about their mean positions. This explains the rigidity in solids. It is due to strong intermolecular forces. A solid may be defined as a form of matter in which the ions, atoms or molecules are held strongly that they cannot easily move away from each other. Hence solid is a rigid form of matter which has a definite shape and a definite volume.
Characteristics of Solid State:
- Solids have a definite shape and volume. Generally, their density is also definite
- Solids are heavier than liquids and gases. (Exception: ice is lighter than water).
- There are strong cohesive forces (inter-particle forces) between the molecules of solid. The forces between the constituent molecules are stronger than those in liquids and gases.
- The molecules of solid are fixed at one point. Thus they are held tightly at a position by intermolecular forces of attraction. Hence solids can’t be poured. The diffusion of solid is negligible.
- Inter-particle distances are short.
- All pure solids have a characteristic melting point which depends on intermolecular forces of attraction. It is above the room temperature at the atmospheric pressure.
- Most of the solids are hard, incompressible and rigid.
- the vapour pressure of solid is much less than the vapour pressure of liquids at a definite temperature. The particles near the surface may possess sufficient kinetic energy to detach from bulk and enter in the vapour state.
Classification of Solids:
On the basis of the nature of order present in the arrangement of their constituent particles, solids can be classified as crystalline or amorphous
Crystalline Solids:
A crystalline solid is a substance whose constituent particles possess a regular orderly long-range arrangement.
Examples: Sodium chloride, sucrose (cane sugar), diamond, quartz, naphthalene, benzoic acid, copper, potassium nitrate, etc.
A crystalline solid usually consists of a large number of small crystals, each of them having a definite characteristic geometrical shape. The arrangement of constituent particles (atoms, molecules or ions) is ordered and repeats itself periodically over the entire crystal.
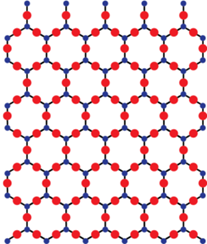
Characteristics of Crystalline Solids:
- A crystalline solid usually consists of a large number of small crystals, each of them having a definite characteristic geometrical shape.
- Crystalline solids have a sharp melting point.
- Crystalline solids are anisotropic in nature, that is, some of their physical properties like electrical resistance or refractive index show different values when measured along different directions in the same crystals. This arises due to a different arrangement of particles in different directions.
- Crystalline solids can be cleaved along a definite plane, hence we get clean cleavage in case of crystalline solids.

- These are considered as true solids.
- Cooling curves of crystalline solids are not smooth, there are breakpoints in the curve.
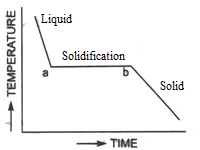
- The heat of fusion is definite and depends upon the arrangement of the particles in the crystalline solids.
Formation of Allotropes:
When a substance exists in two or more forms then they are called allotropes. They are polymorphous.
Example: Diamond and graphite are allotropes of carbon.
Some substances take a different arrangement of particles in the solid-state giving rise to different crystal structures. These different crystalline forms of a substance are known as polymorphic forms or polymorphs. Carbon exists in two crystalline forms diamond and graphite. Sulphur exists in different polymorphic forms, like monoclinic sulphur and rhombic sulphur.
A phenomenon in which when a single substance crystallizes in two or more forms under different conditions of solidification is called polymorphism and the crystals showing polymorphism are called polymorphous.
Examples: Silica forms many polymorphs such as ∝-quartz, ß-quartz, tridymite, cristobalite, coesite, and stishovite.
A phenomenon in which two or more crystalline substances show same crystalline structure is called isomorphism and the crystals showing isomorphism are called isomorphous.
Examples: a) K2SO4 and K2SeO4 and b) NaCl and KCl
Melting of Crystalline Solids:
When a crystalline solid is heated slowly, the temperature of the solid rises gradually until it reaches the melting point. At the melting point, it liquifies suddenly. On cooling the liquid formed, the regular geometric shapes appear again and solid is formed.
Sharp Melting Point of Crystalline Solids:
In a crystal, the arrangement of constituent particles (atoms, molecules or ions) is ordered which repeats itself periodically over the entire crystal. Due to a regular pattern, the interatomic and intermolecular forces are identical. Hence the thermal energy required to break these bonds (i.e. regular structure) is uniform throughout. Hence the heat and temperature required to melt crystalline solid is uniform. Hence crystalline solids have sharp melting points.
Amorphous Solids:

An amorphous solid (Greek Omorphe = no form or shapeless) consists of particles of irregular shape. A substance whose constituent particles do not possess an orderly arrangement is called an amorphous solid.
Examples: glass, plastics, rubber, starch, Teflon, polyvinyl chloride (PVC), polyurethane, etc.
The arrangement of constituent particles (atoms, molecules or ions) in such a solid has only short-range order. In such an arrangement, a regular and periodically repeating pattern is observed over short distances only. Such portions are scattered.
Amorphous solids in many respects resemble liquids which flow very slowly at room temperature and can be considered as supercooled liquids in which cohesive forces are almost as strong as solids.
Due to the short-range order of regular pattern in amorphous solids, the small part of it behaves like crystalline solid. These small parts of amorphous solid behaving like crystalline solid are called crystallites.
Characteristics of Amorphous Solids:
- The arrangement of constituent particles (atoms, molecules or ions) in such a solid has only short-range order.
- The melting point is not sharp. They melt over a range of temperature and can be moulded and blown into various shapes.
- Amorphous solids are isotropic like liquids. It is because there is no long-range order in them and arrangement is irregular along all the directions. Therefore, the value of any physical property would be the same along any direction
- We get irregular cleavage in case of amorphous solids.

- These are pseudo solids or supercooled liquids.
- The cooling curves of amorphous solids are smooth, without any break

- The heat of fusion is not definite.
Uses of Amorphous Solids:
- Amorphous silica is used in a photovoltaic cell which has the capacity to convert quantum energy from light into electrical energy.
- Glass is amorphous solid, which has wide applications in everyday life and also has industrial uses.
- Chemistry of amorphous substance is used in the manufacturing concept of plastic, rubber, ceramics.
- An unbreakable glass is an amorphous substance.
No Sharp Melting Point of Amorphous Solids:
The arrangement of constituent particles (atoms, molecules or ions) in such a solid has only short-range order. Due to the irregular pattern, the interatomic and intermolecular forces are different at different places. Hence the thermal energy required to break these bonds (i.e. regular structure) is not uniform throughout.
Hence the heat and temperature required to melt crystalline solid are not uniform. Hence amorphous solids do not have sharp melting points.
Polycrystalline Solids:
Some solids like aluminium and steel have a structure which is intermediate between crystalline and amorphous. They contain crystal consisting of small units with a definite and regular arrangement but the crystals themselves are randomly arranged.
Note:
Glass panes fixed to windows or doors of old buildings are invariably found to be slightly thicker at the bottom than at the top. This is because the glass flows down very slowly and makes the bottom portion slightly thicker.
Interconversion Between Crystalline and Amorphous Substance
Any crystalline solid can be changed into amorphous form by melting it and cooling the molten mass rapidly. An amorphous substance can be converted into crystalline form by melting it and allowed to cool slowly. Old glasses becomes translucent due to a small extent of crystallization.
Comparative Study of Crystalline and Amorphous Solids:
| Sr.No. | Properties | Crystalline Solids | Amorphous Solids |
| 1 | Structural units | Structural units are arranged orderly and repeating in three dimensions | Structural units are not arranged orderly and repeating in three dimensions |
| 2 | Order | Long order repeating | Short order repeating |
| 3 | Melting points | Very Sharp | Not sharp (melts over temperature range) |
| 4 | Heat of fusion | Definite and characteristics | Neither definite nor characteristics |
| 5 | Cooling curve | Not smooth and with breakpoints | Smooth without a break |
| 6 | Cleavage using knife | Regular and clean cut | Irregular cut |
| 7 | Compressibility | Generally incompressible | Slightly compressible |
| 8 | Nature of bulk | Anisotropic | Isotropic |
| 9 | Nature | True solids | Supercooled liquids or pseudo solids |
Terminology:
Isotropy:
The ability of amorphous solids to exhibit identical physical properties when measured in different directions is called isotropy. This property is due to no long-range order of regular pattern arrangement in them. Thus the arrangement is irregular.
Anisotropy:
The ability of crystalline solids to change their physical properties when measured in different directions is called anisotropy. Some of the physical properties of such solids like electrical resistance or refractive index show different values when measured along different directions in the same crystals. This arises from the different arrangement of particles in different directions.

The electrical resistance of anisotropic solid will be different along line AB and along line CD
Glass:
Glass is an optically transparent material produced by fusing together silicon oxide with sodium oxide, boron oxide. Trace of transition metal oxide is added to impart colour to the glass. By changing composition 800 types of glasses are produced. Quartz is obtained from silicon oxide only.
The composition of different types of glasses is as given below
- Pyrex glass: 60 to 80 % SiO2, 10 to 20 % B2O3, remaining Al2O3.
- Soda lime glass : 75% SiO2, 15 % Na2O, 10 % CaO
The Different oxides used to impart colour to glass are Gold, copper (Red), UO2 (Yellow), CaO, CuO (Blue) and Fe2O3, CuO (Green).
Ice is lighter than water:
The structure of liquid water and solid ice is identical. Ice has a hexagonal three-dimensional crystal structure formed due to intermolecular hydrogen bonding. It leaves about 50 % space vacant. On melting the hydrogen bond between the molecules break and free molecule occupy the empty space which is not utilized in solid ice. Thus the volume of the formed liquid is quite less than the volume of the ice. Thus the density of liquid water is more than that of solid ice. i.e. solid ice is lighter than liquid water.
One reply on “Introduction to Solid State”
Thanks for your support💪💪💪