Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > sp2 Hybridization
Mixing of one ‘s’ orbital and two ‘p’ – orbitals of nearly same energy forming set of trigonally arranged three identical orbitals of equal energy is known as sp2 hybridization.
Geometry sp2 Hybridization:
The hybrid orbitals are arranged around the central atom in a plane at an angle of 120° to one another. When these three orbitals overlap with appropriate orbitals of three other atoms, three bonds are formed and the resulting molecule has a trigonal planar structure. Each bond angle is 120°.
Diagram:
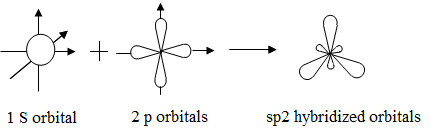
Formation of Boron Trifluoride Molecules:
Ground State of Boron Atom:
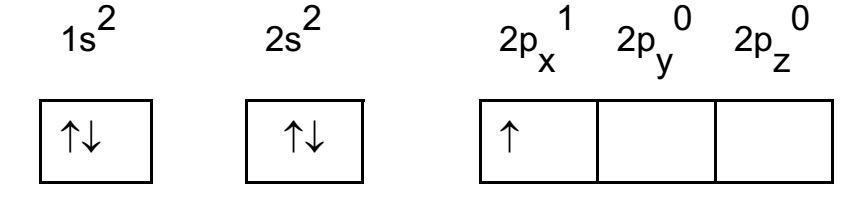
Atomic number of boron is 5. Its configuration in ground state is 1s2, 2s2, 2p1
Boron atom in ground state:
Excited State of Boron Atom:
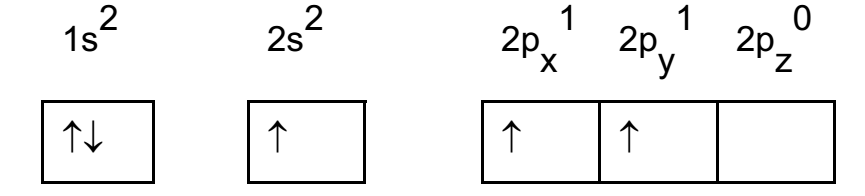
During combination with fluorine, the 2s electron pair is split up and one electron is promoted to empty 2py orbital. This condition is called excited state of boron. In excited state one electron of 2s migrates to 2p orbital forming 3 – half filled orbitals.
Boron atom in excited state:
Hybridization of Boron Atom:
One 2s orbital and two 2p orbitals of boron mix up forming three hybrid orbitals of equivalent energy. These three new equivalent orbitals are called sp2 hybrid orbitals. They are identical in all respect
Boron atom in hybridized state:

Angle and Geometry:
- Three sp2 hybridized orbitals formed, repel each other and they are directed towards the three corners of an equilateral triangle. The angle between them is 120°.
- Each sp2 hybrid orbital contains an unpaired electron.
- In each sp2 hybrid orbital, one of the lobes is bigger because of more concentration of electron density. Only bigger lobe is involved in bond formation.
- Thus BF3 molecule has a trigonal planar structure with boron atom at the centre and three fluorine atoms at the four corners of equilateral triangle. F-B-F bond angle is 120°.
Bond Formation:
Three sp2 hybrid orbitals of boron atom having one unpaired electron each overlap separately with 2p orbitals of three fluorine atoms along the axis forming three covalent bonds (sigma). Thus in BF3 molecule has a planar structure with a boron atom at the centre and three fluorine atoms at the three corners of an equilateral triangle. F-B-F bond angle is 120°.
Bond:
- There are three sigma bonds..
- The bonds between boron and fluorine are sp2– p.
- Thus F – B — F bond angles are 120°. The molecule is trigonal planar.
- All B-F bonds in boron trifluoride are of equal strength.
Diagram:

Type and Geometry of Boron trifluoride Molecule:
| Name of Molecule | Boron trifluoride |
| Molecular Formula | BF3 |
| Type Of Hybridization | sp2 |
| Geometry | Trigonal planar |
| No. Of Bonds | 3 |
| No. Of Sigma bonds | 3 sigma |
| Bond angle | 1200 |
| Overlaps | 3 sp2 – p |
| Bonds | 3 B-F |
The BF3 molecule has a planar structure while NH3 molecule has a pyramidal structure.
During formation of boron trifluoride, boron undergoes sp2 hybridization and achieve the electronic configuration 1s2, 2s12px12py1. In the excited state, one 2s orbital and two 2p orbitals of boron mix up forming three hybrid orbitals of equivalent energy. These three new equivalent orbitals are called sp2 hybrid orbitals. They are identical in all respect. Three sp2 hybridized orbitals formed, repel each other and they are directed towards the three corners of an equilateral triangle (in one plane). Angle between them is 120o. Three sp2 hybrid orbitals of boron atom having one unpaired electron each overlap separately with 1p orbitals of three fluorine atoms along the axis forming three covalent bonds (sigma bonds). Thus boron trifluoride has triangular planar structure.
During formation of ammonia nitrogen undergoes sp3 hybridization. One 2s orbital and three 2p orbitals of mix up forming four hybrid orbitals of equivalent energy. These four new equivalent orbitals are called sp3 hybrid orbitals. They are identical in all respect and they should be directed towards the four corners of a regular tetrahedron and Angle between them should be 109.5o. Three hybridized orbitals contain unpaired electron. The fourth hybridized orbital has lone pair of electron. The three half filled (containing unpaired electron) sp3 hybrid orbitals of nitrogen overlap axially with three half filled 1s orbitals of three hydrogen atoms separately to form three covalent N-H bonds (sigma bonds). The fourth hybrid orbital containing lone pair of electron remains non bonded. The non bonding electron repel each other strongly and occupy more space than the electron pairs involved in bonding. The force of repulsion between lone pair- bond pair is greater than bond pair – bond pair. Hence the
bond angle i.e. H-N-H angle decreases from109.5o to 107o.
Formation of Ethylene Molecule:
Ground State of Carbon Atom:
Atomic number of carbon is 6. Its configuration in ground state is 1s2, 2s2, 2p2 i.e. 1s2 2s2, 2px1 2py1 2pz0
Carbon atom in ground state:

Excited state of Carbon Atom:
During combination with hydrogen, the 2s electron pair is split up and one electron is promoted to empty 2pz orbital. This condition is called excited state of carbon. In excited state one electron of 2s migrates to 2p orbital forming 4 – half filled orbitals.
Carbon atom in excited state:

Hybridization of Carbon Atom:
In ethylene there is sp2 hybridization. One 2s orbital and two 2p orbitals of carbon mix up forming three hybrid orbitals of equivalent energy. These three new equivalent orbitals are called sp2 hybrid orbitals. They are identical in all respect. One ‘p’ orbital of each carbon atom remains unhybridized
Carbon atom in hybridized state:

Angle and Geometry:
Three sp2 hybridized orbitals formed, repel each other and they are directed in a plane towards the three corners of an equilateral triangle. Angle between them is 120o. The unhybridized pz orbital remain perpendicular to this plane. Each sp2 hybrid orbital contain unpaired electron. In each sp2 hybrid orbital, one of the lobes is bigger because of more concentration of electron density. Only bigger lobe is involved in bond formation.
As all the six atoms in C2H6 molecule being in the same plane, the molecule is planar. H-C-H bond angle is 120o. H-C-C bond angle is 120o.
Bonds Formation:
1. Sigma Bond Formation :
A covalent bond formed by collinear or coaxial or in the line of internuclear axis. Overlapping of orbitals is known as sigma bond. One Sp2 hybrid orbital of one carbon atom overlaps with One hybrid orbital of other carbon atom by head on collision forming sigma bond. One (Sp2– Sp2 ) overlap.
Remaining two hybrid orbitals of each carbon atom overlap with ‘s’ orbital of four hydrogen atoms separately forming four sigma bonds. (C – H). Four (Sp2– s) overlaps. All C-H bond in ethylene are of equal strength.
Thus there are five sigma bonds. Sigma bonds are stronger.
2. Formation of pi Bond:
The covalent bond formed by collateral or sidewise overlapping is called pi bond. The unhybridized 2 pz orbitals of each carbon atom being perpendicular to the plane of four hydrogen atoms and carbon atoms overlap laterally with one another to form a week pi bond between two carbon atoms by p – p overlap. One (p-p) -pi bond. This bond consists of two equal electron cloud one lying above the plane of the atom and other lying below this plane.
Hence, in ethylene molecule there are 5 sigma bonds and 1 pi bond.
Diagram :

Type and Geometry of Ethylene Molecule :
| Name of Molecule | Ethylene |
| Molecular Formula | C2H4 |
| Type Of Hybridisation | Sp2 |
| Geometry | Trigonal planar |
| No. Of Bonds | 6 |
| No. Of Sigma bonds | 5 sigma |
| No. of pi Bonds | 1 |
| Overlaps | One (Sp2– Sp2 ) – sigma bond Four (Sp2– s) – sigma bond One (p-p) – pi bond |
| Bond angle | H-C-C 1200 and H-C-H 1200 |
| Overlaps | 4 sp3 – s |
| Bonds | 4 C-H Single Bond ( 4 sigma) 1 C-C Double bond ( 1 sigma 1 pi) |