Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > sp Hybridization
The mixing of one s – orbital and one p – orbital of the same atom of nearly same energy to form a set of diagonally arranged two identical hybrid orbitals of equivalent energy is called sp hybridization. These hybrid orbitals are arranged in a linear manner around central atom and are at an angle of 180o to one another.
Formation of BeF2 Molecule:
Ground State of Beryllium Atom:
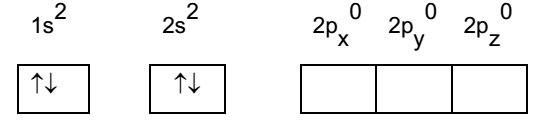
Atomic number of beryllium is 4. Its configuration in ground state is 1s2, 2s2, 2p0.
Beryllium atom in ground state:
Excited state of Beryllium Atom:
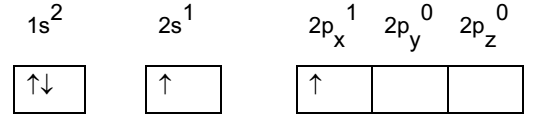
During combination with fluorine, the 2s electron pair is split up and one electron is promoted to empty 2px orbital. This condition is called excited state of beryllium. In excited state one electron of 2s migrates to 2p orbital forming 2 – half filled orbitals.
Beryllium atom in excited state:
Hybridization of Beryllium Atom:
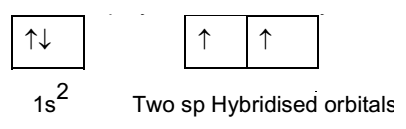
One 2s orbital and one 2p orbitals of beryllium mix up forming two hybrid orbitals of equivalent energy. These two new equivalent orbitals are called sp hybrid orbitals. They are identical in all respect.
Beryllium atom in excited state:
Angle and Geometry :
Two sp hybridized orbitals formed, repel each other and These hybrid orbitals are arranged in a linear manner around central beryllium atom and are at an angle of 180o to one another. Each sp hybrid orbital contain unpaired electron. In each sp hybrid orbital, one of the lobes is bigger because of more concentration of electron density. Only bigger lobe is involved in bond formation.
Thus in BeF2 molecule has linear or diagonal structure with boron atom at the centre and two fluorine atoms at the either sides of beryllium. F-Be-F bond angle is 180o.
Bond:
Two sp hybrid orbitals of boron atom having one unpaired electron each overlap separately with 1p orbitals of two fluorine atoms along the axis forming two covalent bonds (sigma bonds). The bonds between beryllium and fluorine are sp- p overlap. Thus F – Be — F bond angles are 180o. Molecule is diagonal or linear. Both Be-F bonds in beryllium difluoride are of equal strength.
Diagram:

Type and Geometry of Beryllium difluoride Molecule:
| Name of Molecule | Beryllium difluoride |
| Molecular Formula | BeF2 |
| Type Of Hybridisation | Sp |
| Geometry | Diagonal or Linear |
| No. Of Bonds | 2 |
| No. Of Sigma bonds | 2 sigma |
| Bond angle | 1800 |
| Overlaps | 2 sp – p |
| Bonds | 2 Be-F |
BeF2 Is linear molecule, while H2O is angular molecule.
In BeF2 molecule, beryllium undergoes sp hybridization and achieve electronic configuration 1s2, 2s12px1 in excited state. During formation of beryllium difluoride boron undergoes sp hybridization One 2s orbital and one 2p orbital of beryllium mix up forming two hybrid orbitals of equivalent energy. These two new equivalent orbitals are called sp hybrid orbitals. They are identical in all respect. Two sp hybridized orbitals formed, repel each other and they are directed diagonally opposite in space. Angle between them is 1800. Two sp hybrid orbitals of boron atom having one unpaired electron each overlap separately with 1p orbitals of two fluorine atoms along the axis forming two covalent bonds (sigma bonds). Thus BeF2 Is linear molecule.
In water, the oxygen atom is sp3 hybridized. Two hybrid orbitals have paired electrons (lone pair) and they are non – bonding orbitals. Other two orbitals are half – filled (singly occupied) and they are bonding orbitals. These hybridized orbitals are in four directions of four corners of tetrahedron. Four sp3 hybridized orbitals formed, repel each other and they should be directed towards the four corners of a regular tetrahedron and Angle between them should be 109.50. The non bonding electron repel each other strongly and occupy more space than the electron pairs involved in bonding. The force of repulsion between electron pair decreases in following order. lone pair – lone pair > lone pair- bond pair > bond pair – bond pair. Hence the bond angle between two lone pairs i.e. H-O-H angle decreases from109.50 to 104.50. Thus water molecule is angular.
Formation of acetylene (C2H 2) Molecule:
Ground State of Carbon Atom:
Atomic number of carbon is 6. Its configuration in ground state is 1s2, 2s2, 2p2 i.e. 1s2 2s2, 2px1 2py1 2pz0
Carbon atom in ground state:

Excited state of Carbon Atom:
During combination with hydrogen, the 2s electron pair is split up and one electron is promoted to empty 2pz orbital. This condition is called excited state of carbon. In excited state one electron of 2s migrates to 2p orbital forming 4 – half filled orbitals.
Carbon atom in excited state:

Hybridization:
In acetylene there is sp hybridization. One 2s orbital and one 2p orbitals of carbon mix up forming two hybrid orbitals of equivalent energy. These two new equivalent orbitals are called sp hybrid orbitals. They are identical in all respect. Two ‘p’ orbitals of each carbon atom remains unhybridized.

Angle and Geometry:
Two sp hybridized orbitals formed, repel each other and These hybrid orbitals are arranged along x- axis in a linear manner around central carbon atom and are at an angle of 1800 to one another. The unhybridized py and pz orbital remain perpendicular to hybrid orbitals along y-axis and z- axis ,
mutually perpendicular. Each sp hybrid orbital and unhybrid orbitals contain unpaired electron. In each sp hybrid orbital, one of the lobes is bigger because of more concentration of electron density. Only bigger lobe is involved in bond formation.
As all the four atoms in C2H2 molecule being in the same line, the molecule is diagonal or linear. H-C-C bond angle is 180o.
Formation of Bonds:
- Sigma Bond Formation :
A covalent bond formed by collinear or coaxial or in the line of internuclear axis. Overlapping of orbitals is known as sigma bond. One Sp hybrid orbital of one carbon atom overlaps with One hybrid orbital of other carbon atom by head on collision forming sigma bond. One (Sp- Sp ) overlap. Remaining one hybrid orbitals of each carbon atom overlap with ‘s’ orbital of two hydrogen atoms separately forming two sigma bonds. (2 C – H). Two (Sp– s)overlaps. Both C-H bond in Acetylene are of equal strength. Thus there are Three sigma bonds. Sigma bonds are stronger.
2) Formation of pi Bond :
The covalent bond formed by collateral or sidewise overlapping is called pi bond. The unhybridized 2 px and 2 pz orbitals of each carbon atom being perpendicular to each other and to the plane of H-C-C-H axis overlap laterally with one another to form two week pi bond between two
carbon atoms by two p – p overlap. (one 2 py -2 py )and (one 2pz-2 pz) . Thus two (p-p) -overlaps.
In ethylene molecule there are 3 sigma bonds and 2 pi bonds. There is a triple bond between carbon and carbon consisting one sigma and two pi bonds.
Diagram:

Type and Geometry of Acetylene Molecule:
| Name of Molecule | Acetylene |
| Molecular Formula | C2H2 |
| Type Of Hybridisation | Sp |
| Geometry | Diagonal or Linear |
| No. Of Bonds | 5 |
| No. Of Sigma bonds | 3 |
| No. of pi Bonds | 1 |
| Overlaps | One (Sp- Sp) – s bond Two (Sp– s) – s bond Two (p-p) – p bond |
| Bond angle | H-C-C 1800 |
| Bonds | C-H Single Bond (2 sigma) C-C Tripple bond (1 sigma and 2 pi) |
There is only one pi bond in ethylene molecule but there are two pi bonds in the acetylene molecule:
In ethylene molecule carbon atom shows sp2 hybridization in excited state. The resulting three hybrid orbitals form two C-H bonds and One pi bond of sigma type. Thus each carbon atom is left with unhybridized pz orbitals with lobes above and below the plane of hybridized orbitals. These two unhybridized orbitals overlap each other laterally and form a single pi bond between two carbon atoms.
In acetylene molecule carbon atom shows sp hybridization in excited state. The resulting two orbitals are linear and form one C-H bond and one C-C bond of sigma type. Thus each carbon is left with two unhybridized py and pz orbitals, which are mutually perpendicular to H-C-C-H axis. These unhybridized orbitals overlap each other laterally and form two pi bonds between two carbon atoms.