Science > Chemistry > Physical Chemistry > Ionic Equilibria > Hydrolysis Constant
In this article, we shall study hydrolysis of different types of salts and we shall derive expression for hydrolysis constant for each type of salt.
Hydrolysis of salt of strong acid and weak base.
These salts on hydrolysis produce strong acids and weak bases. The resulting solution is acidic in nature.
Consider hydrolysis of ammonium chloride (NH4Cl). It gives strong acid HCl and weak base NH4OH, when treated with water and equilibrium exist as,
NH4Cl + H2O ⇌ NH4OH + HCl
(weak base ) (strong acid)
Applying ionic theory
NH4+ + Cl– + H2O ⇌ NH4OH + H+ + Cl–
Cancelling common ions of both the sides
NH4+ + H2O ⇌ NH4OH + H+
This solution contains free H+ ion. It is acidic to litmus and pH < 7.
Note:
Some salts like CuSO4, FeCl3 on hydrolysis do not give clear solution but give turbid solution. It is due to formation of insoluble hydroxides.
Expression for hydrolysis constant for a salt of strong acid and weak base.
Let one mole of a salt BA of strong acid and weak base be dissolved in water and solution is made Vdm3. The hydrolysis of salt takes place as follows
BA + H2O ⇌ BOH + HA
(weak base ) (strong acid)
Applying ionic theory
B+ + A- + H2O ⇌ BOH + H+ + A-
Cancelling common ions of both the sides
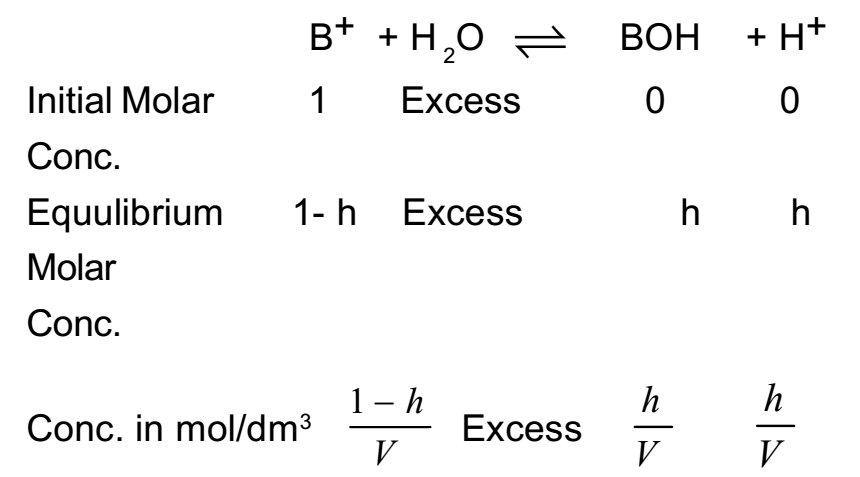
By applying the law of mass action to above equilibrium,
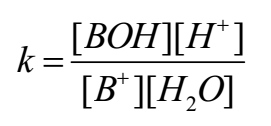
As water is in large excess [H2O] = constant
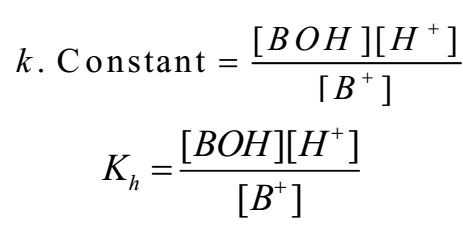
Where Kh is constant called hydrolysis constant.

For a salt of strong acid and weak base h is very small. Hence , 1 – h = h

Thus, the degree of hydrolysis of a salt of strong acid and weak base is inversely proportional to the square root of concentration and directly proportional to the square root of dilution.
Relation between hydrolysis constant (Kh) of salt of strong acid and weak base and dissociation constant(Kb) of a weak base.
The hydrolysis of a salt of strong acid and weak base, BA leads to an equilibrium.
B+ + H2O ⇌ BOH + H+

Besides above equilibrium there are two more equilibria.
Weak base BOH which ionizes to small extent.
BOH ⇌ B+ + OH–

Where Kb is dissociation constant for the weak base.
Water is slightly ionized.
H2O ⇌ H+ + OH –
kw = [H+][OH–] ……….. (3)
Where kw is ionic product of water
Dividing equation (3) by (2), we get

Thus hydrolysis constant of a salt of strong acid and weak base is a ratio of ionic product of water and dissociation constant of a weak base.
Expression of concentration of [H+] in aqueous solution of strong acid and weak base.
The hydrolysis of a salt of strong acid and weak base, BA leads to an equilibrium.

Hydrolysis of salt of weak acid and strong base.
These salts on hydrolysis produce weak acids and strong bases. The resulting solution is basic in nature.
Consider hydrolysis of sodium acetate (CH3COONa). It gives weak acid CH3COOH and strong base NaOH, when treated with water and equilibrium exist as,
CH3COONa + H 2O ⇌ CH3COOH + NaOH
(weak acid) (strong base)
Applying ionic theory
CH3COO– + Na+ + H2O ⇌ CH3COOH + Na+ + OH–
Cancelling common ions of both the sides
CH3COO– + H2O ⇌ CH3COOH + OH–
This solution contains free OH- ions. It is basic to litmus and pH > 7.
Expression for hydrolysis constant for a salt of weak acid and strong base.
Let one mole of a salt BA of weak acid and strong base be dissolved in water and solution is made Vdm3. The hydrolysis of salt takes place as follows
BA + H2O ⇌ BOH + HA
(strong base ) (weak acid)
Applying ionic theory
B+ + A– + H2O ⇌ B+ + OH– + HA
Cancelling common ions of both the sides

By applying the law of mass action to above equilibrium,

As water is in large excess [H2O] = constant

Whre Kh is constant called hydrolysis constant.

For a salt of strong acid and weak base h is very small. Hence, 1 – h = h

Thus, the degree of hydrolysis of a salt of weak acid and strong base is inversely proportional to the square root of concentration and directly proportional to the square root of dilution.
Relation between hydrolysis constant (Kh) of salt of weak acid and strong base and dissociation constant (Ka) of a weak acid.
The hydrolysis of a salt of weak acid and strong base, BA leads to an equilibrium.
A– + H2O ⇌ OH– + HA

Besides above equilibrium there are two more equilibria.
Weak acid which ionizes to small extent.
HA ⇌ H+ + A –

Where Ka is dissociation constant for the weak acid.
Water is slightly ionized.
H2O ⇌ H+ + OH –
kw = [H+][OH–] ……….. (3)
Where kw is ionic product of water
Dividing equation (3) by (2), we get

Thus hydrolysis constant of a salt of weak acid and strong base is a ratio of ionic product of water and dissociation constant of a weak acid.
Expression of concentration of [OH–] in aqueous solution of weak acid and strong base.
The hydrolysis of a salt of strong acid and weak base, BA leads to an equilibrium.

Hydrolysis of salt of weak acid and weak base:
These salts on hydrolysis produce weak acids and weak bases. The nature of resulting solution depends on the relative strengths of the weak acid and the base formed..
Consider hydrolysis of ammonium acetate (CH3COONH4). It gives weak acid CH3COOH and weak base NH4OH, when treated with water and equilibrium exist as,
CH3COONH4 + H2O ⇌ CH3COOH + NH4OH
(weak acid) (weak base)
Applying ionic theory
CH3COO– + NH4+ + H2O ⇌ CH3COOH + NH4OH
As there are no free OH– or H+ ions. The relative strength of CH3COOH and NH4OH are the same. It is neutral to litmus and pH = 7.
Expression for hydrolysis constant for a salt of weak acid and weak base.
Let one mole of a salt BA of weak acid and strong base be dissolved in water and solution is made V dm3. The hydrolysis of salt takes place as follows
BA + H2O ⇌ BOH + HA
(weak base ) (weak acid)
Applying ionic theory

By applying the law of mass action to above equilibrium,

As water is in large excess [H2O] = constant

Where Kh is constant called hydrolysis constant.

For a salt of strong acid and weak base h is very small. Hence, 1 – h = h

Thus, the degree of hydrolysis of a salt of weak acid and weak base is independent of concentration and dilution.
Note:
When h to be found from Kh, use formula

When Kh to be found from h, use formula

Relation between hydrolysis constant (Kh) of salt of weak acid and weak base, dissociation constant(Ka) of a weak acid and dissociation constant(Kb) of a weak base.
The hydrolysis of a salt of weak acid and weak base, BA leads to an equilibrium.
B+ + A– + H2O ⇌ BOH + HA

Besides above equilibrium there are three more equilibria.
Weak acid which ionizes to small extent.

Where Ka is dissociation constant for the weak acid.
Weak base BOH which ionizes to small extent.

Where kb is dissociation constant for the weak base.
Water is slightly ionized.
H2O ⇌ H+ + OH –
kw = [H+][OH–] ……….. (4)
Where kw is ionic product of water
Where KW is ionic product of water
Dividing equation (2) by (3) and (4), we get

.
……… (5)
From equation (1) and (5)