Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > sp3 Hybridization
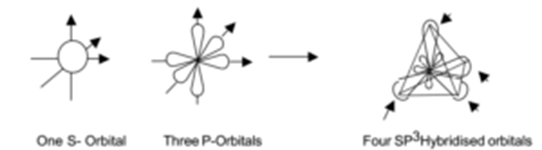
The mixing of one s-orbital and three p- orbitals of the same atom having nearly the same energy to form four orbitals of equal in all respects and tetrahedrally arranged is known as sp3 hybridization.
Geometry of sp3 Hybridization:
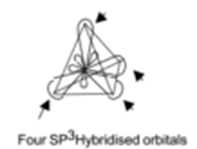
sp3 hybridized orbitals repel each other and they are directed to four corners of a regular tetrahedron. The angle between them is 109.5° and the geometry of the molecule is tetrahedral (non-planar). This type of hybridization is also known as tetrahedral hybridization. The sp3 hybridization is shown pictorially in the figure.
Formation of Methane Molecule ( CH4 ):
Step -1: Formation of the excited state of a Carbon atom:
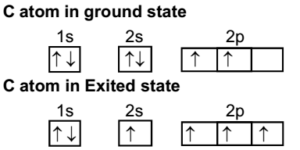
The carbon atom in the ground state takes up some energy and goes to the excited state. In this process, a pair of electrons in 2s orbital splits up and one of the electron from this pair is transferred to empty 2pz orbital. Thus the excited state has four half-filled orbitals.
Step – 2: Hybridization of Orbitals:
One orbital of 2s and three orbitals of 2p mix up forming four hybrid orbitals of equivalent energy. These four new equivalent orbitals are called sp3 hybrid orbitals. They are identical in all respect.

Angle and Geometry:
Four sp3 hybridized orbitals formed, repel each other and they are directed towards the four corners of a regular tetrahedron. The Angle between them is 109.5°. Each sp3 hybrid orbital contains one unpaired electron.
In each sp3 hybrid orbital, one of the lobes is bigger because of more concentration of electron density. Only a bigger lobe is involved in bond formation. Due to this maximum overlapping is achieved.
Four sp3 hybrid orbitals of carbon atom having one unpaired electron each overlap separately with 1s orbitals of four hydrogen atom along the axis forming four covalent bonds. Thus in CH4 molecule has a tetrahedral structure with a carbon atom at the centre and four hydrogens at the four corners of a regular tetrahedron. H-C-H bond angle is 109.5°.
Bonds:
Four sp3 hybrid orbitals of carbon atom having one unpaired electron each overlap separately with 1s orbitals of four hydrogen atom along the axis forming four covalent bonds (sigma bonds). The bonds between carbon and hydrogen are sp3– s. Thus H – C – H bond angles are 109.5°. The molecule is tetrahedral. All C-H bonds in methane are of equal strength.

Type and Geometry of Methane Molecule:
|
Name of Molecule |
Methane |
|
Molecular Formula |
CH4 |
|
Type Of Hybridization |
sp3 |
|
Geometry |
Tetrahedral |
|
No. Of Bonds |
4 |
|
No. Of Sigma bonds |
4 sigma |
|
Bond angle |
109.5° |
|
Overlaps |
4 sp3 – s |
|
Bonds |
4 C-H |
Formation of Ammonia Molecule ( NH3):
Need of Hybridization in Ammonia Molecule:
In nitrogen atom, there are three half-filled 2p orbitals and the valency should be 3 and it is three. These three ‘p’ orbitals are perpendicular to each other and if they form bonds, the angle between them should be 90°. But the actual angle between bonding orbitals in ammonia is 107°. To explain this difference in the angle the concept of hybridization is required.
Electron Configuration:
The Atomic number of Nitrogen is 7. Its configuration in ground state is 1s2, 2s2, 2p3

Hybridization:
In the formation of ammonia one 2s orbital and three 2p orbitals of nitrogen mix up forming four hybrid orbitals of equivalent energy. These four new equivalent orbitals are called sp3 hybrid orbitals. They are identical in all respect. One hybrid orbital has paired electrons (lone pair) and it is nonbonding orbital. The other three orbitals are half-filled and they are bonding orbitals. The nonbonding pair of hybridized orbitals is called as a lone pair. These hybridized orbitals are in the directions of four corners of a regular tetrahedron.

Angle and Geometry:
Four sp3 hybridized orbitals formed, repel each other and they should be directed towards the four corners of a regular tetrahedron and the angle between them should be 109.5°. One hybrid orbital has paired electrons and it is nonbonding orbital. The other three orbitals are half-filled and they are bonding orbitals. But due to greater repulsion exerted by lone pair on bonding orbitals, the angle is reduced to 107°. Thus ammonia has distorted tetrahedral shape.
The three half-filled (containing unpaired electron) sp3 hybrid orbitals of nitrogen overlap axially with three half-filled 1s orbitals of three hydrogen atoms separately to form three covalent N-H bonds Hence geometry is tetrahedral pyramidal in which the nitrogen lies at the centre, three hydrogen atoms form the base and one pair of electrons forms the apex of the pyramid. H-N-H angle is 107°.
Bonds:
The three half-filled (containing unpaired electron) sp3 hybrid orbitals of nitrogen overlap axially with three half-filled 1s orbitals of three hydrogen atoms separately to form three covalent N-H bonds (sigma bonds). The fourth hybrid orbital containing lone pair of the electron remains non bonded. The bonds between Nitrogen and hydrogen are sp3- s. H – N – H bond angles are 107°. All N-H bonds in ammonia are of equal strength.

Type and Geometry:
|
Name of Molecule |
Ammonia |
|
Molecular Formula |
NH3 |
|
Type Of Hybridisation |
sp3 |
|
Geometry |
Pyramidal |
|
No. Of Bonds |
3 |
|
No. Of Sigma bonds |
3 sigma |
|
Bond angle |
107° |
|
Overlaps |
3 sp3 – s |
|
Bonds |
3 N-H |
Formation of Water Molecule:
Need of Hybridization:
In oxygen atom there are two half-filled 2p orbitals and valency should be 2 and it is two. These three ‘p’ orbitals are perpendicular to each other and if they form bonds, the angle between them should be 90°. But the actual angle between bonding orbitals in water is 104.5°. To explain this variation the concept of hybridization is required.
Electron Configuration:
The atomic number of Oxygen is 8. Its configuration in ground state is 1s2, 2s2, 2p4

Hybridization :
In the formation of water molecule one 2s orbital and three 2p orbitals of Oxygen mix up forming four hybrid orbitals of equivalent energy. These four new equivalent orbitals are called sp3 hybrid orbitals. They are identical in all respect. The two hybrid orbitals have paired electrons and they are non – bonding orbitals. Other two orbitals are half-filled and they are bonding orbitals. The nonbonding pairs of hybridized orbitals are called lone pairs. These hybridized orbitals are in the directions of four corners of a regular tetrahedron.

Angle and Geometry:
Four sp3hybridised orbitals formed, repel each other and they should be directed towards the four corners of a regular tetrahedron and the angle between them should be 109.5°. But due to greater repulsion exerted by lone pair on bonding orbitals, the angle is reduced to 104.50. Thus water has distorted the tetrahedral shape.
The two half-filled (containing unpaired electron) sp3 hybrid orbitals of oxygen overlap axially with two half-filled 1s orbitals of two hydrogen atoms separately to form three O-H bonds. Thus geometry is angular or V-shaped, in which the oxygen lies at the centre, two hydrogen atoms occupy two corners of the tetrahedron and lone pair of electrons occupy remaining two corners of the tetrahedron. H-O-H angle is 104.50.
Bonds:
The two half-filled (containing unpaired electron) sp3 hybrid orbitals of oxygen overlap axially with two half-filled 1s orbitals of two hydrogen atoms separately to form two O-H bonds (sigma bond). The remaining two hybrid orbitals containing a lone pair of the electron remains nonbonded.

Type and Geometry:
|
Name of Molecule |
Water |
|
Molecular Formula |
H2O |
|
Type Of Hybridisation |
sp3 |
|
Geometry |
Angular, V -shaped |
|
No. Of Bonds |
2 |
|
No. Of Sigma bonds |
2 sigma |
|
Bond angle |
104.5° |
|
Overlaps |
2 sp3 – s |
|
Bonds |
2 O-H |
5 replies on “sp3 Hybridization”
This is very helpful thank you so much
This was quit helpful, thanks
This is excellent
This was well explained.Thanks a lot guys.
Wow, this is very informative. Thanks