Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > Valence Bond Theory
Valence bond theory was proposed by Heitler and London in (1927) and it was extended by Pauling and Slater (1931). It is based on the following concepts.
- The pairing of electrons.
- Neutralization of opposite electron spins.
- Overlapping of orbitals containing unpaired electrons to give a region of common electron density to the combining atoms.
Postulates of Valence Bond Theory:
Condition of bond formation:
A covalent bond is formed when the atomic orbital of one atom overlaps the atomic orbital of the other atom. The sharing of electrons takes place due to the overlapping of half-filled orbitals of the outermost shell of the two atoms. Only atomic orbital with unpaired electrons with opposite spin can participate in overlapping or bonding. They are known as Bonding Orbitals.
Binding force of covalent bond:
During the overlapping of half-filled orbitals, spins get neutralized and electron density between the two nuclei increases. As a result of this, the force of repulsion between the two nuclei decreases, while the force of attraction between the nucleus of one and the electron of other increases.
Strength of a bond:
Bond is formed when the system attains a stable lowest energy level. This occurs at a certain minimum equilibrium distance between two nuclei known as bond length. The strength of the covalent bond depends upon the extent of overlapping between two atoms. Greater the overlap, stronger is the bond. Complete overlapping is not possible as there are repulsive forces between two nuclei.
Number of covalent bonds:
One bonding orbital can form only one covalent bond. Hence the number of bonding orbitals restricts the number of covalent bonds that can be formed by an atom. The number of unpaired electrons possessed by an atom determines its valency.
Directional nature of covalent bond:
S- orbitals are non-directional but p, d, and f – orbitals have a particular direction. Due to their directional nature, the geometry of the molecule depends upon the orientation of the overlapping of the orbitals.
Geometry of a Molecule on the Basis of Valence Bond Theory:
A covalent bond is formed when the atomic orbital of one atom overlaps the atomic orbital of the other atom. The bond has maximum electron density in the region of overlap. The line joining the nuclei of two atoms determines the direction of the bond.
‘S’ orbitals are spherical and non – directional, but p, d, f orbitals are directionally oriented and produce overlap in the internuclear axis resulting in a bond formation having s a specific direction. The geometry of molecules and bond angles therein are determined by the directional orientation of orbitals involved in the overlap. The electron pairs in the valence shell of the central atom arrange themselves symmetrically so as to get as far apart as possible due to repulsion between them.
e.g. Methane has tetrahedral geometry, Boron trifluoride has planar geometry while Beryllium difluoride has linear geometry, etc.
Energy Changes in Covalent Bond Formation on the Basis of Valence Bond Theory:
Interactive Force Between Approaching Molecules:
It must be realized that when any two atoms approach close to each other new forces of attraction and repulsion set in.
The forces of attraction are between the nucleus of one atom and the electron of the other and vice – versa. The forces of repulsion are between two nuclei amongst themselves as well as between the electrons of the two atoms amongst themselves. The force of attraction and repulsion are represented in the figure.
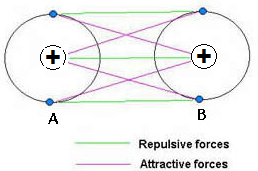
If the net result is attraction i.e. attractive forces are stronger than the repulsive forces the total energy of the system decreases and a chemical bond results. If the net result is repulsion i.e. the repulsive forces are stronger than attractive forces, the total energy of the system increases and no chemical bonding is not possible.
Variation in Energy of System Due to Approach of Orbitals for Overlapping:
When two Hydrogen atoms with unpaired electrons with parallel spin approach each other, repulsion is greater than attraction and energy of system increases and bonding does not take place. In this case, the energy goes on increasing as inter-nuclear distance starts decreasing.
When two Hydrogen atoms with unpaired electrons with opposite spin approach each other, the attraction is greater than repulsion and energy of system continuously decreases till it becomes minimum at equilibrium distance between the two nuclei. There is overlap and leads to covalent bond formation between two hydrogens.
Formation of Hydrogen Molecule in terms of Decrease of Potential Energy:
The way in which the potential energy of the system changes as two hydrogen atoms having electrons with opposite spins approaches each other to form a covalent bond is represented in the potential energy curve. Graphical representation of the change in potential energy as a function of internuclear distance is known as the Potential Energy Curve. The electronic configuration o the hydrogen atom is 1s1. It contains one unpaired electron in its valence shell.
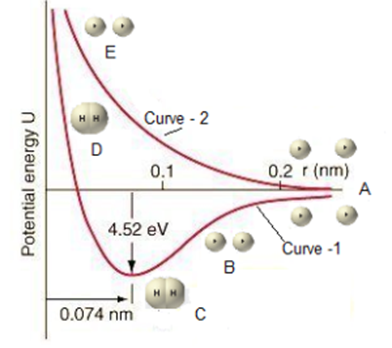
Explanation of Curve – 1
- State A: When two hydrogen atoms are far apart from each other, then the potential energy of one atom is independent of the other. This potential energy is taken arbitrarily as zero
- State B: As the two hydrogen atoms having electrons with opposite spins approach each other, the electrons of one atom begin to feel the attractive forces of the nucleus of the other atom. The attractive force dominates the repulsive force between the electrons. Thus, as the distance between two hydrogen atoms decreases the potential energy of the system gradually decreases.
- State C: A state of minimum potential energy is reached when the forces of attraction are balanced by the forces of repulsion between two hydrogen atoms. At this stage orbital of two hydrogen atoms, overlap and spins of electrons are neutralized and a stable covalent bond is formed between hydrogen atoms, forming a hydrogen molecule. In this case, the overlap of orbitals is maximum. The minimum equilibrium distance between the two hydrogen nuclei at this stage is called bond length which is 74 picometer. The amount of energy released at this stage is called bond energy and it is equal to 431.8 kJ/mole.8.
- State D: When two hydrogen atoms are forced to come still closer, then the potential energy of the system shows a sharp rise. This is due to the increases of repulsive forces between the two nuclei at such small inter-nuclear distance and hence bond formed becomes unstable.
Explanation of Curve – 2
- State E: It two hydrogen atoms having electrons with parallel spins approach each other, potential energy of system increases and no bond is formed between the two hydrogen atoms.