Science > Physics > Themodynamics > Change of Phase
In this article, we should study the concept of phase, change of phase anfd the triple point of water.
Phase:
The phase of a substance is defined as its form which is homogeneous, physically distinct and mechanically separable from other forms of the substance. The term phase as used in thermodynamics refers to the fact that the matter exists either as a solid, liquid or gas. If we consider the example of water, it exists in the solid phase as ice, in the liquid phase as water and in the gaseous phase as vapour. All the substances can exist in any of the three phases under proper conditions of temperature and pressure.
Change of Phase:
The transitions from one phase to another takes place by the absorption or liberation of heat and usually by a change in volume and at a constant temperature. The temperature at which a phase change occurs also depends on pressure.
Phase Diagram:
The phase diagram is the graph drawn in which pressure is represented along y-axis and temperature is represented on the x-axis.
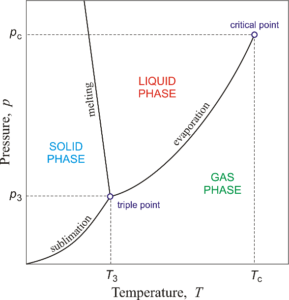
Phase diagram give the relationship between the phase in equilibrium in a system as a function of temperature, pressure and compositions. Phase diagrams are also known as Equilibrium diagrams or Constitutional diagrams.
A phase diagram indicates the temperature at which the solid will start and finish melting and the possible phase changes which will occur as a result of altering the composition or temperature.
The common point, where three lines of phases intersect is known as the triple point. At this point, the substance co-exists in equilibrium in all the three phases i.e. solid, liquid and vapour.
Characteristics of Phase Diagram:
- Different phases of a substance can be shown in a phase diagram.
- A region on a phase diagram represents a single phase of the substance, a curve represents an equilibrium between two phases and a common point represents an equilibrium between three phases.
- A phase diagram helps to determine the condition under which the different phases are in equilibrium.
- A phase diagram is useful for finding a convenient way in which desired change in phase can be produced
Phase Diagram for Water:
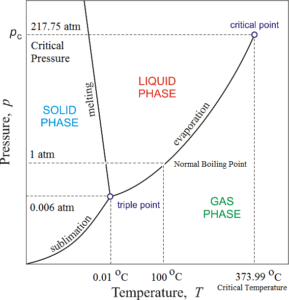
Phase diagram of water consists of three curves sublimation curve, evaporation curve and melting curve meeting each other at a point called triple point. Due to these curves, the phase diagram has three regions
The region to the left of melting curve and above the sublimation curve represents the solid phase of water i.e. ice. The region to the right of melting curve and above the evaporation curve represents the liquid phase of water i.e. water. The region below sublimation curve and evaporation curve represent the gaseous phase of water i.e. vapours.
A curve on the phase diagram represents the boundary between two phases of the two substance. Along any curve, the two phases can coexist in equilibrium.
Along melting curve, ice and water can remain in equilibrium. This curve is called fusion curve or ice line. This curve indicates that the melting point of ice decreases with increase in pressure. Along evaporation curve, water vapours and water can remain in equilibrium. This curve is called vaporisation curve or steam line. This curve indicates that the boiling point of water increases with increase in pressure. Along sublimation curve, ice and water vapours can remain in equilibrium. This curve is called sublimation line or hoar frost line.
The three curves meet each other at a single point at A. This common point is known as the triple point of water. At the triple point of water can coexist in all the three states in equilibrium. The triple point of water corresponds to a pressure of 0.006023 atmospheres and temperature (0.01 °C) 273.16 K.
Significance of Triple Point of Water:
- Triple point temperature of the water is the temperature at which water can coexist in all the three states viz. Ice (solid), water (liquid), vapours (gas) in equilibrium.
- This triple point temperature of the water is used for defining the absolute temperature scale. In absolute or Kelvin scale 0 K is considered as the lower fixed point while the triple point temperature of the water is taken as the upper fixed point.
- Thus one kelvin temperature corresponds to 1/273.16 of the triple point temperature.
Concept of Latent heat
The heat absorbed or released by a substance during the change of its physical state at constant temperature is called latent heat of the substance for that physical change.
Latent Heat of Fusion:
The quantity of heat required to convert unit mass of a solid into liquid state completely at its melting point and at constant temperature is called as latent heat of fusion.
Latent Heat of Vapourization:
The quantity of heat required to convert unit mass of a liquid into gaseous state completely at its boiling point and at constant temperature is called as latent heat of vapourization.
Concept of Internal and External Latent Heat:
Change of state of a substance is always accompanied by increase in the volume. Hence we can say that during the change of state there is always external work done. Thus the latent heat (L) supplied is used for two purposes.
The first part is used to do internal work i.e. to do the work against inter molecular force of attraction to increase the distance between the molecules. This part of latent heat is called internal latent heat. It is denoted by Li.
The second part is used to do external work i.e. to do the work against the external atmospheric pressure to increase the volume of the gas. This part of latent heat is called external latent heat. It is denoted by Le.
L = Li + Le
But the external work done is given by
Le = P ∆V
Where P is a pressure and ∆V is change in the volume
Thus L = Li + P ∆V
This is the relation between internal latent heat and external latent heat.
Characteristics of Internal Latent Heat:
- The part of total latent heat which is used to do external work against intermolecular forces to increase the separation between the molecules is called internal latent heat.
- It increases the separation between molecules of the substances.
- It is greater than external latent heat.
- It is equal to the change in internal energy.
Characteristics of External Latent Heat:
- The part of total latent heat which is used to do external work against the external atmospheric pressure is called external latent heat.
- It increases the volume of the substances.
- It is less than external latent heat.
- It is equal to external work done.