Science > Physics > Heat Transfer > Conduction
Heat always gets transferred from the body and higher temperature to a body at lower temperature heat transfer can take place in three ways a) Conduction b) Convection and c) Radiation. In this article, we shall study the heat transfer by the conduction.
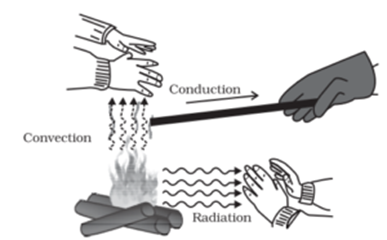
Conduction:
If one end of a metal rod is heated, the other end also gets heated up. This is due to conduction. When one end of a metal rod is heated, the kinetic energy of the molecules at that end increases. The molecules start vibrating with a higher amplitude. These molecules start vibrating with a higher amplitude. These molecules during vibration collide with the neighbouring molecules and transfer part of their energy to the neighbouring molecules. Thus the kinetic energy of the neighbouring molecules increases hence their amplitude of vibration increases and during the collision the energy transfers to the next molecule. Thus heat transfer takes place by conduction.
The mode of heat transfer between two parts of a body or between two bodies in contact which are at different temperatures without actual migration of particles of the body is called conduction.
Depending upon easiness of heat transfer by conduction the substance are classifieds into types a) Good Conductors and b) Bad conductors
Good Conductors:
The substances which allow the heat to pass through them very easily are called good conductors. Examples. Aluminum, copper, Silver, Steel, Bronze, Brass, all metals
Bad Conductors:
The substances which do not allow the heat to pass through them are called bad conductors. Bad conductors of heat are also called as insulators. Examples: wood, rubber, Plastic, paper, glass, air, ebonite , bakelite.
Use of conduction:
- Metals are used for making utensils because the metals are good conductors of heat they allow heat to pass through them easily.
- Cooking vessels have plastic handles because plastic a bad conductor of heat it does not allow the heat to pass through from hot vessel to hands and thus danger of burning can be avoided.
- Tea-cups, Teapots, coffee jugs are made of porcelain.
- Mountaineers use sleeping bags in polar regions.
- People wear woolen cloth in winter.
- Nowadays cooking vessels are made with copper bottoms.
- In winter, the metal lock feels colder than the wooden door on touch.
Characteristics of conduction:
- In this type of heat transfer, there is no actual migration of the medium particles from one point to another.
- For conduction, there must be a material contact between the two bodies.
Concept of Steady-State and Temperature Gradient:
Heat conduction may be described quantitatively as the time rate of heat flow in a material for a given temperature difference.
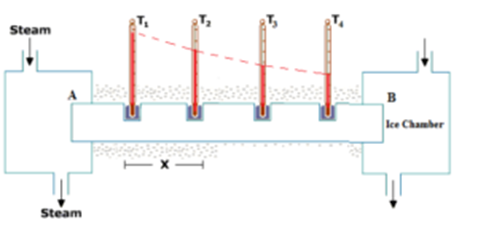
Consider a metallic bar AB of length L and uniform cross-sectional area A with its two ends maintained at different temperatures. The temperature difference between the ends can be obtained by keeping the ends in thermal contact with large reservoirs having temperature differences. Some holes are drilled on this rod to insert thermometers (say T1, T2, T3, and T4) in the rod. For better thermal contact between the rod and thermometers mercury is poured into the holes. The sides of the bar are fully insulated so that no heat is exchanged between the sides and the surroundings.
Let θ1, θ2, θ3, and θ4 be the temperatures recorded by the thermometers T1, T2, T3, and T4 respectively. Initially, the temperature rises and after some time every thermometer shows its own constant reading such that (θ1 > θ2 > θ3> θ4). This state is called the steady-state.
Due to the insulation of the rod, no heat is lost due to surroundings. At a steady-state, at every cross-section of the rod, the quantity of heat entering the section in one second is equal to the quantity of heat leaving the section due to conduction.
Let us consider two sections separated by distance Δx and let Δθ be the temperature difference between these two sections. then the quantity Δθ / Δx is called the temperature gradient.
The temperature gradient is defined as the rate of change of temperature with the distance when the material is in steady-state.
Thermal Conductivity:
It is found experimentally that in this steady state, the rate of flow of heat (or heat current)H is proportional to the temperature difference (θ2 – θ1) and the area of cross-section A and is inversely proportional to the length L
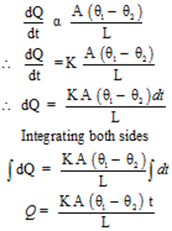
Where K = Constant called the thermal conductivity or the coefficient of thermal conduction the material. The greater the value of K for a material, the more rapidly will it conduct heat.

The SI unit of K is J S–1 m–1 K–1 (joule per second per metre per kelvin) or W m –1 K–1 (watt per metre per kelvin).
The value of thermal conductivity varies slightly with temperature but can be considered to be constant over a normal temperature range. Good thermal conductors have very high values of thermal conductivity while thermal insulators have negligible values of thermal conductivity.
Houses made of concrete roofs get very hot during summer days because the thermal conductivity of concrete (though much smaller than that of metal) is still not small enough. Therefore, a layer of earth or foam insulation is put on the ceiling so that heat transfer is prohibited and the room remains cooler.
Searle’s Experiment:

Apparatus:
Apparatus consists of the thermally insulated box housing a metallic bar of a uniform cross-sectional area with its one end kept in contact with steam in a steam chamber. Two holes are drilled to insert thermometers T1 and T2, in the rod separated by distance x. For better thermal contact between the rod and thermometers mercury is poured into the holes. Cooling water is circulated around the rod whose initial and final temperatures are measured by the thermometers T3 and T4.
Working and Calculations:
At steady state, the heat lost by rod = heat gained by the water

Where, mW = Mass of water, SW = Specific heat of water, t = time for which heat is flowing
Measuring all values on R.H.S. of the formula value of K can be found.
Values of thermal conductivity in J S–1 m–1 K–1 for different materials are given below
| Metals | |
|
Silver Copper Aluminium Brass Steel Lead Mercury |
406 385 205 109 50.2 34.7 8.3 |
| Non-metals | |
|
Insulating brick Concrete Body fat Felt Glass Ice Glass wool Wood Water |
0.15 0.8 0.20 0.04 0.8 1.6 0.04 0.12 0.8 |
| Gases | |
|
Air Argon Hydrogen |
0.024 0.016 0.14 |