Science > Physics > Nuclear Physics > Discovery of Proton and Neutron
Before the discovery of the atomic nucleus, there were ideas that all atoms are composed of hydrogen atoms (called by William Prout “protyles”). This hypothesis is known as Prout’s hypothesis. According to this hypothesis, the hydrogen atom was the only truly fundamental particles, and that the other atoms were actually groupings of various numbers of hydrogen atoms (protyles). In this article, we shall study the discovery of proton and neutron, their characteristics and importance.
Discovery of Proton:
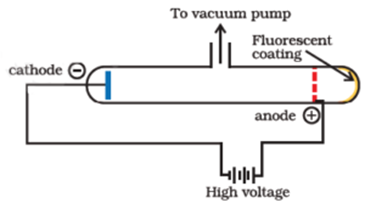
Proton was discovered by E. Goldstein in 1886. He performed the same experiment as performed by J.J. Thomson but used perforated cathode. He found that on passing an electric discharge through a gas under reduced pressure, rays containing positive particles move towards the cathode. As they appear to arise from the anode they are called anode rays or canal rays. They are found to contain positively charged particles called protons.
Origin of Positive Rays:
When an electric discharge is passed through gas at very low pressure in the discharge tube cathode rays are produced. The cathode rays consist of a stream of high-speed electrons. When these fast-moving electrons strike the atoms or molecules of the gas present in the discharge tube, they remove one or more electrons from the neutral atoms or molecules. Thus positive ions of gas are formed. These ions which move towards the perforated cathode kept midway in the tube and constitute the positive rays coming through the perforated cathode. In 1907 a study of deflection of these rays in a magnetic field revealed that the particles making up the ray were not all the same mass. The lightest ones, formed when there was some hydrogen gas in the tube, were calculated to be about 1840 times as massive as an electron.
Characteristics of Canal Rays:
- They travel in a straight line in the opposite direction to that of cathode rays.
- Canal rays produce fluorescence when incident on zinc sulphide screen.
- Unlike cathode rays, the positively charged particles depend upon the nature of gas present in the cathode ray tube.
- These are simply the positively charged gaseous ions.
- The charge to mass ratio of the particles is found to depend on the gas from which these originate.
- Some of the positively charged particles carry a multiple of the fundamental unit of electrical charge.
- The behaviour of these particles in the magnetic or electrical field is opposite to that observed for electron or cathode rays.
Characteristics of Protons:
- Protons are positively charged.
- Protons are located in the nucleus.
- Protons have mass 0f 1.0078 a.m.u. (1.672 × 10-27 Kg.). This mass of a proton is considered as unit mass (1 a.m.u.).
- Mass of one proton is almost equal to the mass of one hydrogen atom.
- The proton carries a positive charge of 1.6 × 10-19 C. This charge carried by the proton is considered to be the unit positive charge.
- Proton is denoted by 1H1 or 1P1. I.e. it has a unit positive charge and unit mass.
- All atoms contain protons.
- Baryons are massive particles that are made up of three quarks in the standard model. The proton is a baryon and is considered to be composed of two up quarks and one down quark.
Discovery of Neutron:
Since the time of Rutherford, it had been known that the atomic mass number A of nuclei is a bit more than twice the atomic number Z for most atoms and that essentially all the mass of the atom is concentrated in the relatively tiny nucleus. In 1920 Rutherford proposed the existence of the third neutral particle in an atom. But up to 1930 proton-electron hypothesis was accepted.
An experimental breakthrough came in 1930 with the observation by the German nuclear physicist Herbert Becker and Walther Bothe that bombardment of beryllium with alpha particles from a radioactive source produced neutral radiation which was penetrating but non-ionizing. They observed that the penetrating radiation was unaffected by electric fields and hence, they assumed it to be gamma radiation. In the year 1932, Frederic Joliot-Curie and Irene Joliot-Curie demonstrated that these rays have the potential to eject protons when it strikes paraffin or any H-containing compounds. The experiment proved that the assumption that the rays to be gamma rays was wrong. Because a photon that does not have mass cannot be capable to release a particle 1836 times heavier than an electron (protons). Therefore, it was concluded that the ejected rays cannot be photons.
Chadwick’s Experiment:
Neutron was discovered by Sir James Chadwick in 1932. He performed the same experiment performed by Frederic Joliot-Curie and Irene Joliot-Curie and used different bombardment targets other than paraffin.
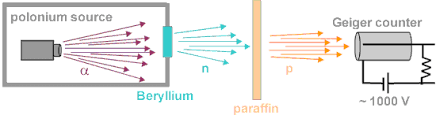
He fired alpha radiation at the beryllium sheet from a polonium source. This led to the production of an uncharged, penetrating radiation. These radiations were made incident on paraffin wax, having relatively high hydrogen content. The range of the liberated protons was measured and the interaction between the uncharged radiation and the atoms of several gases was studied by Chadwick. The particle ejected was found to have a mass equal to that at proton and no charge. He called these particles as neutrons.
4Be9 + 2α4 ⟶ [6C13] ⟶ 6C12 + 0n1
Characteristics of Neutrons:
- Neutrons have no charge i.e. they are electrically neutral.
- They are located in the nucleus of an atom.
- Mass of neutron is of 1.008665 a.m.u (1.675 × 10-27 Kg ). For practical purposes, this mass is assumed as unit mass.
- Mass of neutron is nearly as that of the proton.
- Neutron is denoted as 0 n 1.
- Baryons are massive particles which are made up of three quarks in the standard model. The neutron is a baryon and is considered to be composed of two down quarks and one up quark.
Importance of Discovery of Neutron:
- After the discovery of the neutron, every chemical element present in the periodic table was modified and written accordingly.
- With Chadwick’s announcement, Heisenberg then proposed the proton-neutron model for the nucleus.
- It carries no electric charge, it is used as a projectile in nuclear reaction allowing it to split the nuclei of even the heaviest elements.
- Neutrons found a wide variety of uses, from examining the structures of different materials to determining water content in soil and treating tumours.
- It is used in fission nuclear reaction in a nuclear reactor for the production of nuclear energy
- It is extensively used in nuclear engineering and research.
2 replies on “Discovery of Proton and Neutron”
thank you
it is very important knowledge for me and other peoples of the world we love the science of CHEMISTRY!!!!!!!!