Science > Physics > Electrostatics > Quantization of Electric Charge
In this article, we shall study the concept of quantization of electric charge and the principle of conservation of electric charges.
The fact that all observable charges are always some integral multiple of elementary charge e = 1.6 × 10-19 C is known as quantization of electric charge.
Thus q = ± ne, where n = 1, 2, 3, …..
e = 1.6 × 10-19 C is the magnitude of the lowest possible charge which is carried by an electron and proton. The cause of the quantization of electric charge is due to the fact that when one body is rubbed with the other, an integral number of electrons are transferred. There is no scientific explanation for quantization of electric charge in electrodynamics theory and modern physics but it can be verified experimentally.
At the microscopic level, Gell-Mann and Zweig postulated that all elementary particles are built out of more elementary constituents called quarks. Protons and neutrons are made up of two types of quarks i) up quarks denoted by ‘u’ carrying charge +2e/3 and ii) down quarks denoted by ‘d’ carrying charge – e/3. According to quark model the composition of proton is (uud) carrying charge (2e/3 +2e/3 – e/3 = e) and that of neutron is (udd) carrying charge (2e/3 – e/3 – e/3 = 0). Till now the existence of quarks is not detected experimentally but their existence is proved indirectly. In future when they are detected experimentally only we have to change the definition of quantization from e to e/3. The idea of quantization will remain the same.
Coulomb is not a Practical Unit or it is Very Large Unit:
Let us consider a body giving 1 billion (109) electrons per second. Let us calculate the time to create a charge of 1 C
We have q = ne
∴ Number of electrons required = n = q/e = 1/1.6 × 10-19 = 6.25 × 1018
Time for obtaining these electrons = t = 6.25 × 1018/ 109
= 6.25 × 109 seconds = 6.25 × 109/ (365 × 24 × 60 × 60) = 198.2 years
This indicates that the coulomb is a very large unit, hence practical units like milicoulomb (mC), microcoulomb(μC), nanocoulomb (nC) are used.
Principle of Conservation of Charges:
Electric charge can neither be created nor be destroyed but it is transferred from one part of a system to another part of the system so that the total charge of an isolated system remains constant.
Illustration – 1:
When a glass rod (electrically neutral) is rubbed with a silk cloth (electrically neutral), the loosely attached valence electrons of the glass rod get transferred to the silk cloth. Thus in case of glass rod becomes electron-deficient and acquires a positive charge, while the silk cloth has the excess of negative charge and acquires a negative charge. The total charge of the system i.e. the glass rod and the silk cloth remains zero.
Illustration – 2:
When a γ ray photon having energy equal or greater than 1.01 MeV passes near very close to the nucleus, the electric field created by the nucleus would annihilate γ rays photon and create a pair of an electron and positron. This phenomenon is known as pair production. It is represented as
γ → e– + e+
We can see that the total charge on either side is equal (zero)
Illustration – 3:
When electron and positron come very close to each other, they disappear forming two γ ray photons each of energy o.51 MeV. This phenomenon is known as annihilation of matter. It is represented as
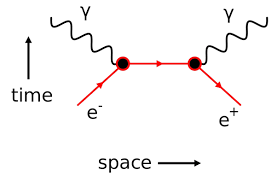
e– + e + → γ + γ
We can see that the total charge on either side is equal (zero)
Illustration – 4:
Consider the following reaction showing α decay of uranium.
92U238 → 90U234 + 2He4
We can see that the total charge on either side is equal (+ 92e)
Illustration – 5:
Consider nuclear fission reaction
92U235 + 0n1 → 156Ba141 + 36Kr92 + 3 0n1 + Energy
We can see that the total charge on either side is equal (+ 92e)
Electric charges have additive nature. The total electric charge on a body is equal to the algebraic sum of all the electric charges located anywhere on the body. When doing the algebraic sum due importance should be given to the sign (positive or negative) should be given.
Numerical Problems:
Example – 01:
How much electronic charge is required to make 1 coulomb.
Given: Total charge = q = 1 C, Electronic charge = e = 1.6 × 10-19 C
To find: Number of electronic charge = n =?
Solution:
We have q = ne
∴ n = q/e = 1/1.6 × 10-19 = 6.25 × 1018
Ans: Number of electronic charge is 6.25 × 1018
Example – 02:
How many electrons should be removed from a conductor so that it acquires a positive charge of 3.5 μC.
Given: Total charge = q = 3.5 μC = 3.5 × 10-6 C, Magnitude of the charge on electron = e = 1.6 × 10-19 C
To find: Number of electrons removed = n =?
Solution:
We have q = ne
∴ n = q/e = 3.5 × 10-6/1.6 × 10-19 = 2.1875 × 1013
Ans: Number of electrons removed is 2.1875 × 1013
Example – 03:
Calculate the positive charge and negative charge on the water in a cup holding 250 g of water.
Given: Mass of water
To find: Number of electrons = n =?
Solution:
The molecular formula for water is H2O. Its molecular mass is 18 g mol-1
Number of Moles of water = Given mass/molecular mass = 250 /18 = 13.89
1 mol of water contains 6.022 × 10²³ molecules of water
Number of molecules in 13.89 moles of water = 13.89 × 6.022 × 10²³
Number of molecules in 13.89 moles of water = 83.66 × 10²³
Each molecule of water contains 2 hydrogens (1 electron each) and 1 oxygen (8 electrons)
Number of electrons in each water molecule = 1 × 2 + 8 × 1 = 10
Total number of electrons in a cup = 83.66 × 10²³ × 10 = 83.66 × 1024
Total negative charge on water = 83.66 × 1024 × 1.6 × 10-19 =1.34 × 107 C
As water is electrically neutral, total positive charge=1.34 × 107 C
Ans: The total negative charge is – 1.34 × 107 C and the total positive charge is + 1.34 × 107 C
Example – 04:
Find the number of electrons moving through an electric bulb per second, rated with power 100 W at 230 V.
Given: Power of bulb = P = 100 W, Voltage = V = 230 V
To find: Number of electrons passed = n =?
Solution:
P = VI =V q/t
∴ q = P t /V = (100 × 1)/230 = 0.4348 C
∴ n = q/e = 0.4348/1.6 × 10-19 = 2.72 × 1018
Ans: Number of electrons passed is 2.72 × 1018
Example – 05:
Two identical spheres carrying charges -2 μC and 14 μC are made to contact each other and then separated. Find charge on each sphere after separation
Given: Charge on first sphere q1 = -2 μC, Charge on the second sphere = q2 = 14 μC
To find: Charge on each sphere =?
Solution:
Total charge on the system = -2 + 14 = 12 μC
As the two spheres are identical the charge will get equally distributed among them
Hence charge on each sphere = 12/2 =6 μC
3 replies on “Quantization of Electric Charge”
The questions were good.
Excellent 👌
more questions should be there