Science > Physics > Radiation > Introduction to Radiation
The different modes of heat transfer are conduction, convection, and radiation. In this article. we shall study radiation.
Radiation:
Radiation is a process of transfer of heat in the form of electromagnetic waves for which material medium is not necessary.
Radiant Heat:
The thermal energy which is transferred by radiation is called radiant heat or radiant heat or simply radiations
Characteristics of Radiation:
- In the process of radiation thermal energy or heat energy is transferred from one point to other in the form of electromagnetic waves.
- As radiation is due to electromagnetic waves and electromagnetic waves are capable of passing through a vacuum, there is no necessity of material medium for radiation.
- Due to the electromagnetic nature of radiation has the same properties as that of light, such as rectilinear propagation, reflection, refraction, interference, etc.
- The velocity of radiant energy in air or vacuum is the same as that of light in vacuum i.e 3 × 108 m/s. Due to this high-speed radiation is the most rapid process of heat transfer.
- When radiant heat is incident on a matter, it is partly absorbed and converted into heat.
- Radiations have the wavelength greater than that of red colour and thus radiation form infrared region of the electromagnetic spectrum.
Relation Between a, r, and t:
Coefficient of Absorption (a):
The coefficient of absorption of a body is defined as the ratio of the quantity of radiant heat absorbed by it to the total quantity of radiant heat incident upon it.
Let Q be the radiant heat incident on the body and Qa be the radiant heat absorbed. Thus,
a = Qa /Q
Coefficient of Reflection (r):
The coefficient of reflection of a body is defined as the ratio of the quantity of radiant heat reflected by it to the total quantity of radiant heat incident upon it.
Let Q be the radiant heat incident on the body and Qr be the radiant heat reflected. Thus,
r = Qr /Q
Coefficient of Transmission (t):
The coefficient of transmission of a body is defined as the ratio of the quantity of radiant heat transmitted by it to the total quantity of radiant heat incident upon it.
Let Q be the radiant heat incident on the body and Qt be the radiant heat transmitted. Thus,
t = Qt /Q
Relation:
Let Q be the total radiant heat incident on the surface of a body. Let Qa, Qr, Qt be the radiant heat absorbed, reflected and transmitted respectively by the body.
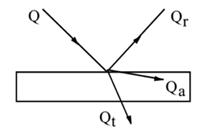
Then, Qa + Qr + Qt = Q
Dividing both sides of the equation by Q
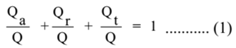
∴ a + r + t = 1
Thus the sum of the coefficient of absorption, the coefficient of reflection and the coefficient of transmission is unity.
Classification of Substances:
Diathermanous Substance:
The substances which can transmit the radiant heat incident upon their surfaces are called diathermanous substances e.g. glass, quartz, gases
Characteristics of Diathermanous Substance:
- The substances which can transmit the radiant heat incident upon their surfaces are called diathermanous substances.
- For such a substance, the value of the coefficient of transmission (t) is not zero.
Adiathermanous Substance:
The substances which cannot transmit the radiant heat incident upon their surfaces are called adiathermanous (athermanous) substances e.g. wood, iron copper, etc.
Characteristics of Adiathermanous Substance:
- The substances which cannot transmit the radiant heat incident upon their surfaces are called adiathermanous (athermanous) substances.
- For such a substance, the value of the coefficient of transmission (t) is zero.
Numerical Problems:
Example – 01:
The coefficients of reflection and transmission of the surface of a thin plate are 0.22 and 0.04 respectively. If 250 J of radiant heat is incident on the surface of the plate, how much heat is absorbed by the surface?
Given: Coefficient of reflection = r = 0.22, coefficient of transmission = 0.04, radiant heat incident = Q = 250 J
To Find: Radiant heat absorbed = Qa =?
Solution:
We have, a + r + t = 1
∴ a + 0.22 + 0.4 = 1
∴ a = 1 – 0.26 = 0.74
∴ Coefficient of absorption = a = 0.74
Now, Qa = aQ = 0.74 × 250 = 185 J
Ans: Radiant heat absorbed = 185 J
Example – 02:
Coefficient of absorption of a body is 0.54 and coefficient of reflection is 0.16. The amount of radiant heat incident on a body is 4000 J. Calculate the amount of heat transmitted through the body.
Given: Coefficient of absorption = r = 0.54, coefficient of reflection = 0.16, radiant heat incident = Q = 4000 J
To Find: Radiant heat transmitted = Qt =?
Solution:
We have, a + r + t = 1
∴ 0.54 + 0.16 + t = 1
∴ t = 1 – 0.70 = 0.30
∴ Coefficient of transmission = t = 0.30
Now, Qt = tQ = 0.30 × 4000 = 1200 J
Ans: Radiant heat transmitted = 1200 J
Emissive Power:
Emissive power of a surface is defined as the amount of heat radiated per unit time per unit area of that surface at a given temperature.

It is found that the emissive power of a perfectly black body is greater than the emissive power of any other surface at the same temperature. It is denoted by the letter ‘E’ and its S.I. unit is J/m²s or W/m².
The emissive power of a body depends on
- the temperature of a body
- the nature of the body
- the surface area of the body
- the nature of the surroundings.
Coefficient of Emission (Emissivity):
The coefficient of emission of a surface is defined as the ratio of heat radiated per unit time per unit area of the surface to the heat radiated per unit time per unit area of a perfectly black surface at the same temperature. OR Coefficient of emission of a surface is the ratio of the emissive power of the surface to the emissive power of a perfectly black surface at the same temperature.

The coefficient of emission is also known as emissivity. It is denoted by letter ‘e’ and it is a unitless quantity. For perfectly black body e = 1, for perfect reflector e = 0 and for all other bodies 0 < e < 1.
Let E be the emissive power of the body and Eb be the emissive power of the perfectly black body at the same temperature, then
E = e Eb = a Eb
Prevost’s Theory of Heat Exchanges:
The exchange of heat between a body and its surroundings is explained on the basis of Prevost’s theory. The main points of Prevost’s heat exchange theory are
- Every body emits and absorbs thermal radiations at all temperatures except at absolute zero.
- The rate at which thermal radiations are emitted depends upon the absolute temperature of the body.
- The rate of radiation is independent of the temperature of the surrounding.
- Case -1: When a body is at a higher temperature than that of the surroundings then it radiates heat at a faster rate than it absorbs. Therefore it loses heat and its temperature falls.
- Case – 2: When a body is at a lower temperature than that of surroundings then it absorbs heat at a faster rate than the rate at which it radiates heat. Therefore, it gains heat and its temperature rises.
- Case – 3: When the temperature of the body is the same as that of the surroundings then the body radiates heat at the same rate at which it absorbs. Therefore, the body neither loses nor gains heat and hence its temperature remains constant. So, even in this state the processes of radiation and absorption continues.
Next Topic: Kirchhoff’s Law of Radiation