Science > Physics > Nuclear Physics > Concept of Radioactive Decay
Transformation of radioactive element into another element (radioactive or non-radioactive) is known as radioactive decay or disintegration. In radioactive decay, the nucleus of a radioactive element called parent undergoes a spontaneous change accompanied by the emission of radiation and the formation of the nucleus of a new element called the daughter. The physical and chemical properties of the daughter may be different from its parent.
There are three types of Radioactive Decay
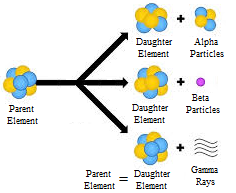
Alpha Decay:
Alpha decay is a type of radioactive decay in which a particle with two neutrons and two protons (Helium nuclei) is ejected spontaneously from the nucleus of a radioactive atom. During alpha decay, an atom’s nucleus sheds two protons and two neutrons. Alpha particles are Helium nuclei.
Alpha decay occurs in very heavy elements (having nucleons 210 or more) like uranium, thorium, and radium. Nuclei of these elements have a large proton to neutron ratio, which makes these elements neutron-rich. This richness makes alpha decay possible. These nuclei are so large that the short-range nuclear forces holding the nucleons together are unable to counterbalance the electrostatic repulsion among the large number of protons in them. Therefore, in an attempt to achieve greater stability by reducing their size, they emit an alpha particle.
When radioactive substance emits one α -particle, the mass number of daughter element formed is 4 units less and the atomic number is 2 units less.
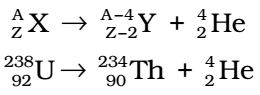
The daughter element (Th) occupies two positions to the left of the parent element (U).
Energy Change and Distribution:
The α-decay process is “fueled” by the rest mass energy difference of the initial state and final state. i.e. the alpha-decay of Uranium can occur spontaneously (without an external source of energy) because the total mass of the decay products and an alpha particle is less than the mass of the original substance uranium. Thus, the total mass-energy of the decay products is less than the mass-energy of the original nuclide. The difference between the initial mass-energy and the final mass-energy of the decay products is called the Q value of the process, or the disintegration energy. Thus, the Q value of an alpha decay can be expressed as
Q = (mX – mY – mHe) c2
Since alpha particles has a high binding energy, its formation within the nucleus causes release of sufficient energy which becomes available for escape. The energy Q is shared by the daughter nucleus Y and the alpha particle. Mostly the energy is taken by the alpha particle.
As per Hans Geiger and John Mitchell Nuttall (Gamow Theory), short-lived isotopes emit more energetic alpha particles than long-lived ones. Or the value of Q increases the half-life period decreases.
Beta decay:
A nucleus that decays spontaneously by emitting an electron or a positron is said to undergo beta decay. When a radioactive substance emits one β -particle, the atomic number of daughter element formed is one unit higher but the mass number remains unchanged.
In 1930 Pauli proposed that during beta decay, the proton in the nucleus is transformed into a neutron and vice versa. Thus there are three types of beta decay
Positron Emission:
If a proton is converted to neutron, by β+ decay. In this conversion neutron to proton ratio increases.

The change is accompanied by emission of antineutrino
Electron Capture
If a proton is converted to neutron, by electron capture. In this conversion neutron to proton ratio increases.

The change is accompanied by emission of neutrino
Positron Emission:
if a neutron is converted to a proton, it is known as β- decay. In this conversion neutron to proton ratio decreases.

The change is accompanied by emission of neutrino
Due to the change in the nucleus, a beta particle is emitted. The beta particle is a high-speed electron when it is a β- decay and a positron when it is a β+ decay.
The symbols nu bar and nu represent antineutrino and neutrino, respectively; both are neutral particles, with very little or no mass. These particles are emitted from the nucleus along with the electron or positron during the decay process. Neutrinos interact only very weakly with matter; they can even penetrate the earth without being absorbed. It is for this reason that their detection is extremely difficult and their presence went unnoticed for long.
The above explanation shows why the mass number A of a nuclide undergoing beta decay does not change; one of its constituent nucleons simply changes its character (proton into neutron or neutron into a proton).
Types of Beta Decay
There are three types of Beta Decay:
Electron Emission
The process of ejection or emission of electron from the nucleus is known as electron emission. After the emission, the charge of the nucleus increases by one.

Electron Capture
Electron capture is the phenomena where the nuclei decay by capturing one of the electrons that surround the nucleus. This leads to a decrease of one in charge of the nucleus.

Positron Emission
It is the third form of beta decay. A positron is an antimatter equivalent of an electron & has the same mass as of an electron, but bares the opposite charge of an electron. Positron decay produces a daughter nuclide with one less positive charge on the nucleus than the parent.

Energy Change and Distribution:
The difference between the initial mass-energy and the final mass-energy of the decay products is called the Q value of the process, or the disintegration energy. Thus, the Q value of an alpha decay can be expressed as
Q = (mX – mY – me) c2
The energy Q is shared by the beta particles, and the antineutrino /neutrino in all proportions with each other. Daughter element being heavy carries negligible energy. When the antineutrino grabs whole of the energy, the beta particle is emitted with zero energy and vice-versa. Thus beta particles come out with a continuous range of energy which remains conserved.
Change in Momentum:
The antineutrino conserves the momentum also. Before the emission of the beta particle, the momentum of the parent nucleus is zero. The antineutrino is emitted along with beta particle with a momentum which is exactly equal to the sum of the momenta of the beta particle and daughter nucleus.
Gamma Decay:
There are energy levels in a nucleus, just like there are energy levels in atoms. Gamma decay is the nucleus’s way of dropping from a higher energy level to a lower energy level through the emission of high energy photons. Most of the time, gamma decay occurs after the radioactive nuclei have undergone an alpha or a beta decay. When a nucleus is in an excited state, it can make a transition to a lower energy state by the emission of electromagnetic radiation. As the energy differences between levels in a nucleus are of the order of MeV, the photons emitted by the nuclei have MeV energies and are called gamma rays. Unlike, alpha decay and beta decay, the parent nucleus does not undergo any physical change in the process, daughter and parent nuclei are the same.
Gamma rays are emitted by the nucleus, particle decay or annihilation reactions. It is to be noted that X-rays are emitted by electrons (either in the orbits or in outside applications like particle accelerators, synchrotrons radiation etc)
Most radionuclides after an alpha decay or a beta decay leave the daughter nucleus in an excited state. The daughter nucleus reaches the ground state by a single transition or sometimes by successive transitions by emitting one or more gamma rays. A well-known example of such a process is that of 27Co60. By beta emission, the 27Co60 nucleus transforms into 28Ni60 nucleus in its excited state. The excited 28Ni60 nucleus so formed then de-excites to its ground state by successive emission of 1.17 MeV and 1.33 MeV gamma rays. This process is depicted in the following energy level diagram.

