Science > Physics > Themodynamics > Introduction
In this article, we shall study the concept of thermodynamics and thermodynamic state. Thermodynamics is a branch of physics that deals with the inter-conversion between heat energy and any other form of energy.
Thermodynamic State:
The simplest example of a system to which thermodynamics can be applied is a single chemically defined homogeneous substance. In this case, the thermodynamic state can be described completely by specifying any two of the three quantities, pressure P, volume V, and temperature T. These quantities are known as thermodynamic parameters or thermodynamic variables of the system.
For a given amount of the substance forming the system, these three quantities are not independent. They are connected by a relationship of the general form which is called equation of state. It is for this reason that any two of these quantities are sufficient to describe the thermodynamic state completely. The two quantities are then called the thermodynamic coordinates.
Terminology of Thermodynamics:
System:
The specified portion of the physical universe under thermodynamic study is called the system. e.g. A gas enclosed in a cylinder fitted with a piston is a system.
Surroundings:
Remaining part of the universe outside the system which can exchange energy with the system and which change the properties of the system is called a surrounding.
Universe:
The system and its surroundings are together known as the universe.
Boundary:
The real or imaginary surface separating the system from the surrounding is called the boundary
Isolated system:
A system which can exchange neither matter nor energy with the surroundings is called isolated system. Example:A liquid placed in a thermos flask is an isolated system. Temperature change outside the flask does not change the temperature of the liquid. (no energy transfer) and nothing can escape from or enter the flask (no transfer of matter). The total amount of energy remains constant. Both the mass and temperature of the system constant.
Homogeneous system:
When a system is a uniform throughout or consists of a single phase, it is said to be the homogeneous system. Example:A pure single solid, liquid or a gas. A mixture of gases.
Heterogeneous system:
A heterogeneous system is one which is not uniform throughout and which contains two or more phases which are separated from one another by definite boundary surface. Example:Two immiscible liquids such as benzene and water
Mechanical Equilibrium:
A thermodynamic system is said to be in mechanical equilibrium if no unbalanced forces and torques act between the system and the surroundings or between different parts of the system.
Thermal Equilibrium:
A thermodynamic system is said to be in thermal equilibrium if the temperature of the system is the same throughout and the temperature of the system and the surroundings is the same.
Chemical Equilibrium:
A thermodynamic system is said to be in chemical equilibrium if the chemical composition of the system is the same throughout.
Thermodynamic Equilibrium:
A thermodynamic system is said to be in thermodynamic equilibrium if it is in mechanical, thermal and chemical equilibrium at the same time.
Equation of a State:
The equation of state for any substance is a mathematical formula which expresses the relationship between the volume, pressure and temperature of the substance in any state of aggregation. Thus, for example, the equation of state for one mole a perfect gas is PV = RT
The equation of the state can be written in the form, f(P, V, T) = 0.
The thermodynamic state of the system can be specified by stating the values of two coordinates. The value of the third variable can be determined by using the equation of state of the system.
Isothermal Process:
A process carried out at a constant temperature throughout the process is called isothermal process.
Isothermal Change:
When a thermodynamic system undergoes a change in its state at a constant temperature, the change is said to be Isothermal change. The condition of Isothermal change is given by dT = 0.
Conditions for Isothermal change:
The isothermal change can take place when the system is contained in a container having perfectly conducting walls due to which heat produced or absorbed during the change will flow out or flow in from the surrounding. Therefore, the temperature of the system remains constant.
In practice, no perfect container is available and therefore perfect occurrence of isothermal change is impossible. However, a fairly approximate isothermal change is obtained when the change is made slowly.
Examples of Isothermal Changes:
- Ice is converted into the water at constant temperature.
- Water is converted into vapours at constant temperature i.e. boiling point of water.
A gas can be allowed to expand or is compressed isothermally by changing the pressure on it.
Isothermals or Isotherm or Isothermal Curves:
A graph of pressure versus volume at constant temperature is called isotherm or isothermal or isothermal curve. D
A graph is drawn by taking pressure on the y-axis and volume on the x-axis. This graph is known as PV diagram or indicator diagram.
Consider a gas occupying a volume v1 at pressure P1. The thermodynamic state of the gas is represented by a point H on the graph. Let us suppose that the gas undergoes an isothermal expansion from H to K along curve HK. Obviously, V2 > V1. During expansion, the gas does an external work and its internal energy decreases. The curve HK is known as Isothermal curve or Isothermal.
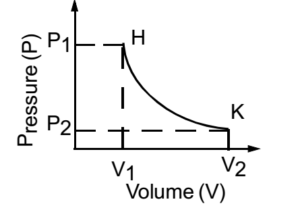
However, the change is made from K to H along curve KH, the gas undergoes an isothermal compression. Therefore, the volume of the gas decreases from V2 to V1. The external work is on the gas hence its internal energy increases.
For one mole of a perfect gas PV = RT or PV = constant. This relation at constant temperature is known as isothermal relation for a perfect gas.