Science > Physics > Themodynamics > Laws of Thermodynamics
In this article, we shall study the laws of thermodynamics and the concept of work done in a process.
Zeroth Law of Thermodynamics:
If two bodies P and Q are in thermal equilibrium and also P and R in thermal equilibrium then Q and R, are also in thermal equilibrium. This law introduces the concept of hotness and coldness which leads to the concept of the temperature of a body.
Characteristics of Thermal Equilibrium:
- When two bodies are kept in contact and there is no transfer of heat taking place between the two bodies, then the two bodies are said to be in thermal equilibrium with each other.
- When two bodies are in thermal equilibrium, there is no heat transfer between the two bodies due to conduction or convection.
- All bodies in thermal equilibrium are at equal temperatures.
- If thermal equilibrium does not exist, then heat flows from a body at a higher temperature to the body at a lower temperature, till thermal equilibrium is established.
First Law of Thermodynamics:
Energy can’t be created nor destroyed but it can be converted from one form into the other (or forms) or into work. The total amount of energy of an isolated system remains constant, it may change from one form to another.
Mathematical Expression of First Law:
ΔQ = ΔU + ΔW
Where ΔQ = Heat supplied to the system by the surroundings
ΔW = Work done by the system on the surroundings
Δ U = Change in internal energy of the system
Process and its Types:
Process:
It is the path or the operation by which a system changes from one state to another. A process causes a change in the value of at least one of the state functions.
Isothermal process:
If a process is carried out at a constant temperature, the process is called an isothermal process. e.g. Fusion of ice.
For an isothermal process, ΔT = 0, ΔU = 0. The internal energy (U) of a system remains constant during the isothermal process provided there is no change of phase.
Characteristics of Isothermal Process.
- In this process temperature of the system remains constant.
- The exchange of heat takes place with the surroundings. ( ΔQ ≠ 0)
- Internal energy remains constant. ΔU = 0 (provided there is no change in a phase).
- The system is not thermally isolated from the surroundings.
- Expansion occurs with the absorption of heat, while compression occurs with the evolution of heat.
- ΔW = ΔQ
- In the case of gases, Boyle’s law is applicable i.e. PV = Constant
Adiabatic process:
A process carried out in such a manner that the system, undergoing the change, does not exchange heat with the surroundings is called an adiabatic process. The temperature of the system changes during the adiabatic process. e.g. expansion of a gas in a vacuum.
Characteristics of Adiabatic Process.
- If a process is carried out in such a manner that the system, undergoing the change, does not exchange with the surroundings is called an adiabatic process.
- The exchange of heat with the surrounding does not take place. ( q = 0)
- Internal energy varies. (ΔU ≠ 0)
- The system is thermally isolated from the surroundings.
- In expansion temperature and internal energy decreases, while in compression temperature and internal energy increase.
- W = ΔU
- In the case of gases, PVγ = Constant, where γ = Ratio of specific heat capacities of a gas
Second Law of Thermodynamics:
Mechanical work can be converted completely into heat but heat can not be completely converted into mechanical work, i.e. work and heat are not equivalent. Thus it is impossible to construct a 100 % efficient engine.
Heat Engines:
A heat engine is a device which takes heat from bodies at a higher temperature, converts part of it to mechanical work and remaining heat is rejected to the body at a lower temperature. The cycle is repeated again and again to get useful work.
Consider working of an internal combustion engine. In the cylinder of the engine, fuel is burned. The gases formed expand to move the piston. The arrangement converts reciprocating motion into rotational motion, which is responsible for the movement of an automobile. On the return stroke of the piston, the gases in the cylinder are expelled to the surroundings.
The efficiency of a heat engine is defined as the ratio of useful work (W) obtained from the heat engine to the heat input to the engine (Qi). Thus
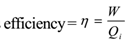
Now work done W = Qi – Q2
Where Qi = Heat input
Q2 = Heat rejected to surroundings
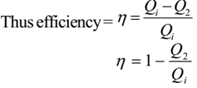
Refrigerators:
The refrigerator is a reverse of heat engines. It is a device which takes heat from bodies at a lower temperature, and heat is rejected to the surroundings at a higher temperature. Hence mechanical work is to be done.
In condenser of refrigerator working fluid (freon gas) is suddenly expanded due to which the mixture of vapour-liquid is formed. This mixture is compressed to a liquid. This liquid is then passed through or around the region to be cooled. This region is called the evaporator. In this region, the liquid is made to evaporate and the necessary heat for evaporation is removed from the region to be cooled. Thus heat is taken out from the body at a lower temperature. This liquid returns back in the condenser, where the heat is rejected to the surroundings which is at a higher temperature than the area to be cooled. Thus the cycle repeats. Mechanical work is to be done on the system, which is done by the compressor.
The coefficient of performance (COP) of a refrigerator is defined as the ratio of heat extracted from the cold reservoir to the work done on the system.
Thus COP = Q2 / W
Now work done W = Qi – Q2
Where Qi = Heat input
Q2 = Heat extracted from cold reservoir
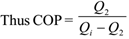
Free Expansion:
When gas is made to expand when there is no external pressure, the expansion of a gas is called the free expansion of the gas. Free expansion of the gas is an irreversible process.
Quasi-Static Process:
The thermodynamic process which takes place infinitely slowly is called a quasi-static process. In practice, there is no process which is perfectly quasi-static. A quasi-static process is reversible and its direction can be reversed at any instant.
Example. Isothermal expansion of gas taking place very slowly in a cylinder fitted with a frictionless and weightless airtight movable piston.