Science > Physics > Thermal Properties of Matter and Thermodynamics > concept of Heat
“Heat” refers to the transfer of thermal energy between substances due to a temperature difference. It’s a form of energy associated with the motion of atoms and molecules in a substance. Heat always flows from regions of higher temperature to regions of lower temperature until thermal equilibrium is reached, where the temperatures become equal. For example, if Object A and Object B are connected, and Object A has a higher temperature than Object B, heat moves from Object A to Object B, causing Object A’s temperature to decrease and Object B’s temperature to increase. This also means that Object A’s average kinetic energy is decreasing and Object B’s average kinetic energy is increasing. This process of transfer of heat will continue till both the objects acquire the same temperature.
Caloric Theory of Heat:
The caloric theory of heat was a scientific hypothesis that dominated the understanding of heat and temperature for several centuries, particularly during the 18th and early 19th centuries. It posited that heat was a fluid-like substance called “caloric” that flowed from hot objects to cold objects, thereby causing changes in temperature. Key principles of the caloric theory include:
- Heat as a Fluid: Caloric was thought to be a weightless, invisible fluid that could be added to or removed from substances to produce changes in temperature. When caloric flowed into an object, it caused the object to become hotter, and when it flowed out, the object became colder.
- Conservation of Caloric: Similar to the conservation of mass or energy, the caloric theory proposed that caloric was a conserved substance. It could change form or location but could not be created or destroyed.
- Explanation of Heat Phenomena: The caloric theory was used to explain various heat-related phenomena, such as the expansion of materials when heated, the melting of solids into liquids, and the evaporation of liquids into gases.
- Phlogiston Theory Connection: The caloric theory was often intertwined with the phlogiston theory, which was another outdated scientific hypothesis related to combustion and the nature of fire. Both theories attempted to explain natural phenomena using concepts of invisible, weightless substances.
The caloric theory faced challenges and criticisms, particularly as scientific understanding advanced and experimental evidence mounted against it. One significant challenge came from the work of James Prescott Joule and others who demonstrated that mechanical work could be converted into heat, suggesting a connection between mechanical energy and heat that couldn’t be explained by the caloric theory alone.
The downfall of the caloric theory began with the experiments and insights of scientists like Benjamin Thompson (Count Rumford) and Antoine Lavoisier, who demonstrated the connection between heat and mechanical work. Ultimately, the caloric theory was replaced by the kinetic theory of gases and the understanding that heat is a form of energy associated with the motion of particles (atoms and molecules) within substances. This transition paved the way for the development of modern thermodynamics and the understanding of heat transfer on a molecular level.
Kinetic Theory of Heat:
According to this theory all substances (solids, liquids, and gases) are made up of molecules. At any given temperature above absolute zero (0 Kelvin or -273.15 degrees Celsius), molecules and atoms of substance are in constant, random motion. This motion arises from their kinetic energy, which increases with temperature. Depending upon the temperature and nature of the substance the molecules may possess three types of motion:
- Translational Motion: Molecules and atoms can undergo translational motion, meaning they move from one location to another. In gases, translational motion dominates, and molecules move freely and independently, colliding with each other and the walls of their container.
- Rotational Motion: Molecules, especially those with multiple atoms, can rotate about their centre of mass. The extent of rotational motion depends on the molecule’s shape and the presence of external influences like electric or magnetic fields.
- Vibrational Motion: Atoms within molecules are connected by chemical bonds, and these bonds can act like springs, allowing atoms to vibrate relative to each other. Vibrational motion occurs at specific frequencies dictated by the strength of the bonds and the masses of the atoms involved.
When a body is heated, the average kinetic energy of its molecules and atoms also increases. This leads to several observable effects on molecular motion:
- Increased Speed: At higher temperatures, molecules and atoms move faster on average. This is because temperature is a measure of the average kinetic energy of particles in a substance. As temperature rises, particles gain kinetic energy and move more rapidly.
- Increased Frequency of Collisions: With higher molecular speeds, the frequency of collisions between molecules and atoms also increases. This results in more energetic interactions and a greater likelihood of chemical reactions occurring, especially in gases and liquids.
- Increased Vibrational and Rotational Motion: In addition to increased translational motion (movement from one place to another), higher temperatures can also increase the vibrational and rotational motion of molecules. This is particularly evident in gases and liquids where molecules have more freedom to move.
- Expansion of Solids, Liquids, and Gases: As temperature rises, the average distance between molecules or atoms increases, causing substances to expand. This expansion is observed in solids, liquids, and gases, although the extent and mechanism vary for each state of matter.
- Changes in Phase: Temperature plays a critical role in phase transitions, such as melting, freezing, boiling, and condensation. As temperature increases, substances can transition from one phase to another by overcoming intermolecular forces or bonds that hold them together in their current state.
The Kinetic Theory provides a basis for understanding heat transfer processes, including conduction, convection, and radiation. Heat transfer involves the transfer of kinetic energy between particles through collisions. The Kinetic Theory of Heat played a crucial role in the development of thermodynamics and statistical mechanics, providing a molecular-level explanation for many macroscopic observations related to the behaviour of gases, liquids, and solids. It also laid the groundwork for modern understanding of heat, temperature, and energy transfer processes.
Heat is Energy in Transit:
“Heat is energy in transit” is a succinct and accurate statement describing the fundamental concept of heat transfer in physics. Heat refers to the transfer of thermal energy between objects or systems due to a temperature difference. It moves from regions of higher temperature to regions of lower temperature until thermal equilibrium is achieved.
In this context, “energy in transit” implies that heat is not a substance itself but rather a form of energy that is transferred from one object to another. When heat flows between objects, it can bring about changes in temperature, phase transitions (like melting or boiling), or other thermal effects.
Units of Heat:
The units of heat can vary depending on the context and the system of measurement being used. CGS Unit of Heat:
CGS unit of heat is calorie (cal). One calorie is defined as the amount of heat required to raise the temperature of one gram of water by one degree celsius (from 14.5 to 15.5 oC) at a pressure of one atmosphere.
Larger unit is kilocalorie (Kcal)
1 Kcal = 1000 cal
Conversions:
| From | to | Multiplying Factor |
| cal | Kcal | X 10-3 |
| Kcal | cal | X 103 |
SI Unit of Heat:
SI unit of heat is joule. One joule is defined as the amount of energy transferred when a force of one newton acts over a distance of one meter in the direction of the force.
1 calorie = 4.186 joule
Conversions:
| From | to | Multiplying Factor |
| cal | J | X 4.186 |
| J | cal | ÷ 4.186 |
Joule’s Mechanical Equivalent of Heat:
The mechanical equivalent of heat is a concept in physics that relates mechanical work to heat energy. It was experimentally determined by James Prescott Joule in the 19th century and contributed significantly to the development of the theory of energy conservation.
Joule’s experiments demonstrated that mechanical work could be converted into heat energy and vice versa, implying that heat and mechanical energy were interchangeable forms of energy. His most famous experiment involved stirring water with paddles inside an insulated container, thereby converting mechanical work into an increase in the temperature of the water.
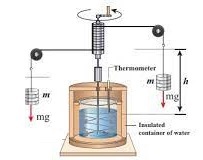
The main component of this experiment is the Joule apparatus. The apparatus basically works as follows. A weight connected to a pulley system is dropped from a known height, thereby turning side pulleys in the system. A center pulley in turn rotates a paddle wheel inside a container containing liquid. The outside of the container is thermally insulated to prevent heat loss from the container. Rotating the paddle wheel causes the temperature of the liquid to rise.
The paddle wheel with its many fins is fitted inside the thermally insulated container. A thermometer is fitted to read change in temperature. The shaft of the paddle wheel is connected through wires to a centre pulley located directly above the paddle wheel and then to two side pulleys, one on each side of the support structure. A weight can be attached to the end of each wire using a hook. The experiment is performed by attaching a known weight to one wire, dropping it a known distance, and then removing the weight. During this event, the wire on the other side is wound up. The experiment continues by attaching another weight and then dropping the weight as before. This process is repeated many times such that a measurable increase in the liquid’s temperature is observed. In Joule’s apparatus, the gain in the paddle wheel’s energy from the energy gained in dropping the weight becomes the gain in the heat energy of the liquid.
It is observed, W α H
Thus, W = JH
∴ J = W/H
The accepted value for the mechanical equivalent of heat is approximately 4.184 joules per calorie, meaning that it takes 4.184 joules of mechanical work to produce 1 calorie of heat energy.
Thus Mechanical Equivalent of Heat
J = 4.186 J cal-1 = 4.186 x 107 erg cal-1
This discovery provided experimental evidence for the principle of conservation of energy, which states that energy cannot be created or destroyed but can only be converted from one form to another. The mechanical equivalent of heat helped unify the concepts of heat, work, and energy, laying the foundation for the development of thermodynamics and modern physics.
Conclusion:
Heat is a form of energy that can be transferred between objects or systems as a result of a temperature difference. It is not a substance itself but rather a mode of energy transfer. Heat is commonly measured in units such as joules (J) in the International System of Units (SI) or calories (cal) in the metric system. Heat can be transferred through conduction, convection, and radiation. Heat can produce various effects on matter, including changes in temperature, phase transitions (such as melting, boiling, and condensation), expansion or contraction of materials, and chemical reactions. Heat plays a central role in the field of thermodynamics, which studies the relationships between heat, work, and energy. The laws of thermodynamics govern how heat behaves and is transferred in various systems. Understanding the principles of heat and heat transfer is crucial in fields such as physics, engineering, chemistry, and meteorology, as heat plays a fundamental role in many natural and technological processes.
For More Topics in Thermal Properties of Matter and Thermodynamics Click Here