Science > Physics > Thermal Properties of Matter and Thermodynamics > Concept of Temperature
In last article we have discussed concept of heat. In this article, we shall study the concept of temperature, different temperature scales, and convert temperature in different temperature scales.
Defining Temperature:
Temperature can be defined in several ways:
- The temperature may be defined as the degree of hotness or coldness of a body.
- It is an indicator of the average thermal energy (Kinetic energy) of the molecules of the body.
- It is that physical quantity which decides the flow of heat in bodies brought in contact. Heat always flow from the body at higher temperature to the body at the lower temperature.
It is measured in °C (centigrade or Celsius) or K (Kelvin). It is measured by a device called a thermometer.
Kinetic Interpretation of Temperature:
Temperature reflects the average kinetic energy of particles in a substance. The kinetic energy of a particle is the energy associated with its motion. According to the kinetic theory:
- Temperature and Kinetic Energy: As the temperature of a substance increases, the average kinetic energy of its particles also increases. This means that at higher temperatures, the particles move faster on average.
- Temperature and Particle Speed: Temperature is directly related to the average speed of the particles in a substance. Higher temperatures correspond to higher average speeds, while lower temperatures correspond to lower average speeds.
- Collisions and Pressure: The kinetic theory also explains pressure in terms of particle motion. When particles collide with the walls of their container, they exert a force, resulting in pressure. The force of the collisions depends on the speed of the particles, which in turn is related to temperature.
- Absolute Zero: According to the kinetic theory, at absolute zero (0 Kelvin), particles would have minimal kinetic energy, meaning they would completely cease their motion. This is the lowest possible temperature and represents the point at which particles have minimal energy.
Thus, the kinetic interpretation of temperature provides a fundamental understanding of how the motion of particles at the microscopic level influences the macroscopic properties of a substance, such as its temperature, pressure, and volume.
Various Temperature Scales:
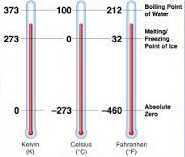
There are several temperature scales used around the world, each with its own reference points and units of measurement. Here are the most common temperature scales:
Celsius Scale (° C):
In this scale, the melting point of ice at one-atmosphere pressure and at mean sea level is taken as the lower reference point and consider as 0° C. While boiling point of water at one atmosphere pressure and at mean sea level is taken as an upper reference point and consider as 100° C. The range between the two reference points is divided into 100 equal parts and each part is called 1° C (one degree Celsius). This scale is also called a centigrade scale.
A lower limit of 0° C is considered arbitrary, this scale can be extended to indicate negative temperatures also. A temperature below -273.15° C is not possible.
Fahrenheit Scale (° F):
In this scale, the melting point of ice at one-atmosphere pressure and at mean sea level is taken as the lower reference point and consider as 32° F. While boiling point of water at one atmosphere pressure and at mean sea level is taken as the upper reference point and consider as 212° F. The range between the two reference points is divided into 180 equal parts and each part is called 1° F (one degree Fahrenheit). Nowadays, this scale is not in use.
Kelvin Scale (K):
In this scale, the lowest possible temperature -273.15° C is taken as a lower reference point. This temperature is called absolute zero. The division of 1 K is equal to 1° C. The unit of temperature in the kelvin scale is K (kelvin) and is considered as the fundamental unit in the S.I. system of units.
Reaumer Scale:
In this scale, the melting point of ice at one-atmosphere pressure and at mean sea level is taken as the lower reference point and consider as 0° R. While boiling point of water at one-atmosphere pressure and at mean sea level is taken as an upper reference point and consider as 80° R. The range between the two reference points is divided into 80 equal parts and each part is called 1° R (one-degree Reaumer).
Difference between Heat and Temperature:
Heat and temperature are related concepts in thermodynamics, but they represent different aspects of thermal energy.
| Heat | Temperature |
| Heat is a form of energy that is transferred between objects or systems due to a temperature difference. | Temperature is a measure of the average kinetic energy of the particles in a substance. It indicates how hot or cold an object or substance is relative to a reference point. |
| It represents total kinetic energy of the molecules of a body. | It represents average kinetic energy possessed by the molecules of a body. |
| It is the transfer of thermal energy from a region of higher temperature to a region of lower temperature. | It determines the direction of heat flow, as heat flows from higher temperature regions to lower temperature regions. |
| Heat is cause because when heat is supplied to a body temperature of the body increases and when heat is removed from the body temperature of the body decreases | It is the effect of addition or removal of heat from the body. |
| Heat is measured in units such as joules (J) or calories (cal). | It is measured in units such as Celsius (°C), Fahrenheit (°F), or Kelvin (K). |
For More Topics in Thermal Properties of Matter and Thermodynamics Click Here