Science > Physics > Gravitation > Numerical Problems on Critical Velocity and Period of Satellite Example – 18: A satellite revolves around a planet of the mean density of 104 kg/m3. If the radius of its orbit is only slightly greater than the radius of the planet, find the time of revolution of the satellite. G […]
Science > Physics > Gravitation > Numerical Problems on Critical Velocity and Period of Satellite In this article, we shall study to solve problems to calculate time period and orbital speed of satellite. Example – 01: Calculate the speed and period of revolution of a satellite orbiting at a height of 700 km above the […]
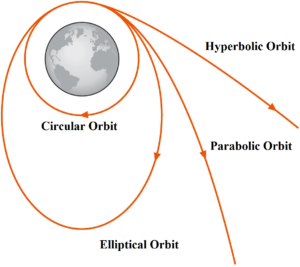
Science > Physics > Gravitation > Critical Velocity and Time Period of Satellite In this article, we shall derive expressions for the critical velocity and time period of a satellite in different forms. Critical Velocity: The constant horizontal velocity given to the satellite so as to put it into a stable circular orbit around the […]
Satellites

Science > Physics > Gravitation > Satellites In this article, we shall study about satellites, their types and uses of artificial satellites Satellites: Any object that revolves around a given planet in circular orbit under the influence of planet’s gravitational force is called as a satellite. Types of Satellites: Satellites are of two types. viz […]
Numerical Problems on Keppler’s Laws
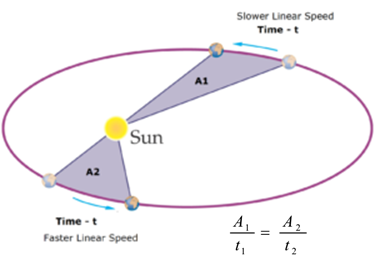
Science > Physics > Gravitation > Numerical Problems on Keppler’s Laws In this article we shall study numerical problems on Keppler’s third law of orbital motion, to calculate period of revolution of a planet around the sun. Example – 01: What would be the length of the year if the earth were at half its […]
Keppler’s Laws of Orbital Motion

Science > Physics > Gravitation > Keppler’s Laws of Orbital Motion In this article, we shall study Keppler’s laws of orbital motion and their use to derive Newton’s law of gravitation. In the second century A.D., Ptolemy proposed a geocentric theory of the universe. According to this theory, the Earth is the centre of the universe and […]

Science > Physics > Gravitation > Variation in Acceleration Due to Gravity The acceleration of gravity is constant at a particular place but it varies from place to place. In this article, we shall study this variation in acceleration due to gravity. Variation in Acceleration Due to Gravity due to Shape of the Earth: The acceleration […]
Acceleration Due to Gravity

Science > Physics > Gravitation > Acceleration Due to Gravity In this article, we shall study the concept of acceleration due to gravity and its characteristics. Also, we shall derive an expression for the same and solve some numerical problems. Weight of a Body: Weight of a body is the force with which the body […]
Concept of Gravitational Potential

Science > Physics > Gravitation > Concept of Gravitational Potential In this article, we shall discuss the concept of the gravitational potential at a point in a gravitational field. It is defined as the work done in bringing the unit mass from infinity to that point without acceleration. Considering magnitude only. VG = W / […]
Concept of Gravitational Intensity

Science > Physics > Gravitation > Concept of Gravitational Field Intensity In this article, we shall study the concept of the gravitational field, its characteristics, the concept of inertial and gravitational mass, and numerical problems on the gravitational field. The Concept of Inertial Mass and Gravitational Mass Inertial Mass: The inertial mass of a body […]