Science > Chemistry > States of Matter > Hydrogen Bonding
Consider two polar molecules water H2O (molecular mass 18 and dipole moment 1.8 D) and nitrosyl fluoride ONF (molecular mass 49 and dipole moment 1.8 D). As their dipole moments are the same, their boiling points should be comparable. But boiling point of water is 100° C while that of nitrosyl fluoride is – 56° C. Thus there is a large difference between their boiling points. This difference is due to a special type of bonding between water molecules called hydrogen bonding.
The large difference in electronegativities of the pairs of bonded atoms N-H, O-H, F-H and Cl-H establish highly polar covalent bonds in which hydrogen acquires exceptionally large positive charge and another electronegative atom acquires exceptionally large negative charge on it. Due to this, there is an attraction between the positively charged hydrogen atom of one molecule and negatively charged atom of another molecule. This interaction is called hydrogen bonding. This concept was introduced by Latimer and Rodebush in 1920. A hydrogen bond is an electromagnetic attraction created between a partially positively charged hydrogen atom attached to a highly electronegative atom and another nearby electronegative atom.
This interaction is represented by a dotted line. The energy of the hydrogen bond varies between 10 to 100 kJ mol-1. This energy is significant. Hence it is a very important factor which influences the bulk properties of a substance.
Variation of Boiling Points of Hydrides:
Following graph shows a variation of boiling points of hydrides of groups 4A, 5A, 6A, 7A.
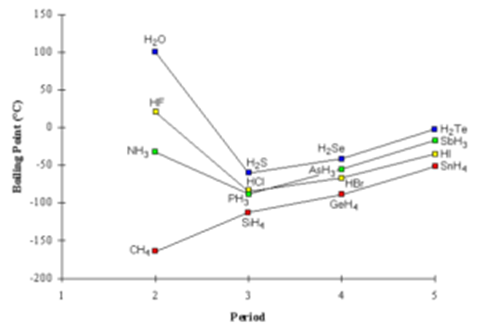
From the graph, we can see that the boiling point of ammonia (hydride of nitrogen), water (hydride of oxygen) and hydrogen fluoride (hydride of fluorine) are exceptionally higher in their group. It is due to strong molecular hydrogen bonding.
We can see that methane (hydride of carbon) has a low boiling point due to the absence of hydrogen bonding. In case of inert gases, the trend is increasing in boiling points with the increase in atomic mass. It is due to dispersion forces increase with the mass of the molecule.
Nature of Hydrogen Bonding:
- A hydrogen bond is a type of dipole-dipole interaction; it is not a true chemical bond it is a mere electrostatic attraction.
- These attractions can occur between molecules (intermolecularly) or within different parts of a single molecule (intramolecularly).
- Intramolecular hydrogen bonds are those which occur within one single molecule. This occurs when two functional groups of a molecule can form hydrogen bonds with each other. In order for this to happen, both a hydrogen donor an acceptor must be present within one molecule, and they must be within close proximity of each other in the molecule. For example, intramolecular hydrogen bonding occurs in ethylene glycol (C2H4(OH)2) between its two hydroxyl groups due to the molecular geometry.
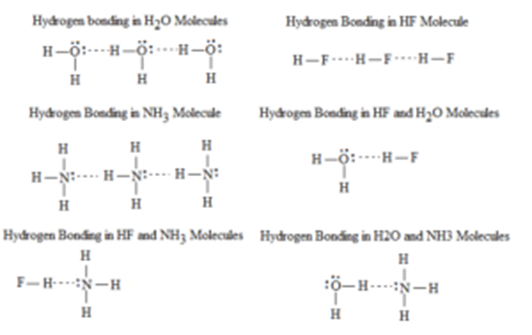
- Intermolecular hydrogen bonds occur between separate molecules in a substance. They can occur between any number of like or unlike molecules as long as hydrogen donors and acceptors are present an in positions in which they can interact. For example, intermolecular hydrogen bonds can occur between NH3 molecules alone, between H2O molecules alone, or between NH3 and H2O molecules.
- Hydrogen bond never involves more than two atoms.
- With the increase in electronegativity and decrease in the size of the atom to which hydrogen is covalently attached, the strength of the bonding increases.
- Hydrogen bonds are generally stronger than ordinary dipole-dipole and dispersion forces, but weaker than true covalent and ionic bonds.
Effects of Hydrogen Bonding:
- Dissociation: Due to hydrogen bonding in HF, in aqueous solution, hydrogen fluoride (HF) dissociates and gives the difluoride ion instead of fluoride ion. The molecules of HCl, HBr, HI do not form hydrogen bonding. This explains the non -existence of compounds like KHCl2 , KHBr2, KHI2.
- Association: The molecules of carboxylic acids exist as dimer because of the hydrogen bonding. The molecular masses of such compounds are found to be double than those calculated from their simple molecular formula.
- High melting and Boiling point, Less Volatility: As extra energy is required to break hydrogen bonds, the compounds having hydrogen bonding show abnormally high melting and boiling points. The unusually high boiling points of hydrogen fluoride (w.r.t other hydrogen halides), water (w.r.t. H2S, H2Se and H2Te), ammonia (w.r.t PH3), ethanol (w.r.t. ethers) are due to the existence of hydrogen bonding.
- Solubility: Solubility increases with the increase in easiness to form a hydrogen bond. Lower alcohols are soluble in water because of the hydrogen bonding which can take place between water and alcohol molecule.
- Viscosity and surface tension: The substances having hydrogen bonding exist as an associated molecule. Hence their flow becomes comparatively difficult. Thus they have higher viscosity and high surface tension.
Importance of Hydrogen Bonding:
- These bonds occur in inorganic molecules, such as water, and organic molecules, such as DNA and proteins.
- The two complementary strands of DNA are held together by hydrogen bonds between complementary nucleotides (A&T, C&G).
- In water it contributes to its unique properties, including its high boiling point (100 °C) and surface tension.
- Intramolecular hydrogen bonding is partly responsible for the secondary, tertiary, and quaternary structures of proteins and nucleic acids. The hydrogen bonds help the proteins and nucleic acids form and maintain specific shapes.