Science > Chemistry > States of Matter > Interaction Between Molecules
Intermolecular forces are the forces of attraction between neighbouring molecules, situated at a distance much closer in comparison with their molecular diameter. They vary from state to state of matter. These forces exist in all states of matter. Liquid and solid states of matter are regarded as a condensed state. These forces arise due to the interaction between molecules. These forces are collectively called as van der Waal’s forces. The intermolecular forces are much weaker than the intramolecular forces. The melting and boiling points of the substances depend on the magnitude of intermolecular forces. Larger the magnitude of these forces higher is the melting point and boiling point of the substance.
These forces due to the interaction between molecules are further classified as a) Dipole-dipole interaction b) Dipole-induced dipole interaction c) Dispersion forces or London Forces. Besides van der Waal’s forces, there are two more intermolecular attraction forces called as ion-dipole interaction and hydrogen bonding.
Dipole-Dipole Interaction Between Molecules:
This effect was studied by Keesom in 1912, hence these forces are also called Keesom forces and also referred as orientation effect. Compounds having atoms of different electro-negativities act as a dipole (e.g. SO2, HCl, NO2, etc.) Dipole-dipole interaction results in a force of attraction between neighbouring molecules having a permanent dipole moment. This interaction is due to the electrostatic force.
Consider polar molecule HCl, a compound of electropositive element hydrogen and electronegative element chlorine. Due to which chlorine pulls shared electron in the bond towards itself acquiring small negative charge (δ–) and hydrogen acquires an equal positive charge (δ+). Thus the HCl molecule becomes an electrical dipole.
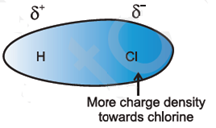
Due to these, all molecules align in a way that oppositely charged ends come close to one another as shown.
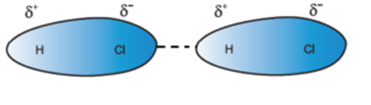
Other examples of this type of interaction are HBr, H2S, NH3.
Characteristics of Dipole-Dipole Interaction:
- Dipole-dipole interaction results in a force of attraction between neighbouring molecules having a permanent dipole moment due to electrostatic force.
- The strength of dipole-dipole interaction depends upon the dipole moment of the interacting molecule.
- Besides dipole-dipole interaction, the polar molecules interact with the London forces. The combined effect of the two increases the total intermolecular forces.
- The dipole-dipole interaction becomes significant when the distance between the dipoles is less than 500 pm.
- In dipole-dipole interactions for mobile dipoles, the interaction energy is inversely proportional to the sixth power of the distance between the two interacting molecules.
Interaction energy ∝ – 1/r6
- The negative sign indicates that energy is released when the two dipoles approach each other.
- In dipole-dipole interactions for stationary dipoles (as in solids), the interaction energy is inversely proportional to the cube of the distance between the two interacting molecules.
Interaction energy ∝ – 1/r3
- The negative sign indicates that energy is released when the two dipoles approach each other.
- The energy for dipole-dipole interaction lies between 4.18 J – 12.55 J
Dipole-Induced Dipole:
This interaction was studied by Debye (1920). This interaction is also called an inductive effect or induction effect. These type of forces operate between polar (μ > 1) and nonpolar (μ = 1) molecules.
In such interaction, the permanent dipole of the polar molecules induces dipole on the nonpolar molecule by deforming or polarizing its electronic cloud.
The interaction energy of these forces is inversely proportional to the sixth power of the distance between the two interacting molecules.
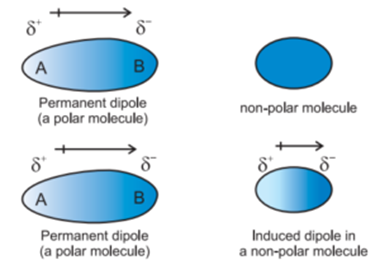
The strength of the forces depends on the distance between the molecules and the polarizability of the nonpolar molecule.
Example: Interaction between NH3 (polar) and C6H6 (nonpolar).
The ease with which an atom or non-polar molecule’s electron cloud can be distorted is called its polarizability. In smaller molecules the electron cloud is near to the nucleus hence the electron cloud cannot be distorted easily. Hence smaller molecules are less polarizable than the larger molecules. In a group of a periodic table, the polarizability increases down the group while in the period the polarizability decreases as we move from left to right in a period of a periodic table.
Characteristics of Dipole-Induced Dipole Interaction:
- In such interaction, the permanent dipole of the polar molecules induces dipole on the nonpolar molecule by deforming or polarizing its electronic cloud.
- The interaction energy of these forces is inversely proportional to the sixth power of the distance between the two interacting molecules.
- The strength of the forces depends on the distance between the molecules and the polarizability of the nonpolar molecule.
- Polarizability decides the ease of condensation of gases containing atoms or non-polar molecules.
Dispersion Forces or London Forces:
This force of attraction was first proposed by the German physicist Fritz London, and for this reason force of attraction between two temporary dipoles is known as London forces. Another name for this force is dispersion force.
Atoms and nonpolar molecules are electrically symmetrical and have no dipole moment because their electronic charge cloud is symmetrically distributed. But a dipole may develop momentarily even in such atoms and molecules due to polarizability. Polarizability is an ease with which the arrangement of electrons in the atom or molecule can be disturbed.
Suppose we have two nonpolar atoms ‘A’ and ‘B’ in the close vicinity of each other. It may so happen that momentarily electronic charge distribution in one of the atoms say ‘A’, becomes unsymmetrical i.e., the charge cloud is more on one side than the other.

This results in the development of instantaneous dipole on the atom ‘A’ for a very short time. This instantaneous or transient dipole distorts the electron density of the other atom ‘B’, which is close to it and as a consequence, a dipole is induced in the atom ‘B’. The temporary dipoles of atom ‘A’ and ‘B’ attract each other. Similarly, temporary dipoles are induced in molecules also.
Characteristics of Dispersion Forces:
- In this interaction, the atoms or nonpolar molecules may develop a dipole momentarily due to polarizability.
- These forces are always attractive and interaction energy is inversely proportional to the sixth power of the distance between two interacting particles. where r is the distance between two particles.
- These forces are important only at short distances (~500 pm) and their magnitude depends on the polarizability of the particle.
- The strength of these forces increases with the increase in molecular mass, molecular size, number of electrons and surface area of the molecule.
- The liquid state of helium and methane is due to the presence of dispersion forces. Other examples are N2, H2, CO2.
- The magnitude of dispersion forces depends on the molecular mass, molecular size, molecular geometry.
- The dispersion forces increase with the increase in the molecular mass and molecular size of an atom. A more spacious arrangement of atoms in molecules results in greater dispersion forces than the compact arrangement.
Ion-Dipole Interactions:
Ion-Dipole Forces are involved in solutions where an ionic compound is dissolved into a polar solvent. Hence the system exhibiting ion-dipole interactions are solutions and not pure substances.
Cations are smaller than anions. The charge on cations is more concentrated. Due to this, the interaction between a cation and the negative end of the polar molecule are stronger than the corresponding interaction between the anion and the positive end of the polar molecule.

Example: Interaction between the ions formed by dissolving NaCl in water and water molecules. ions of NaCl dissociates because of the attraction between the separated ions and oppositely charged poles of water.
Hydration is a process by which each ion is surrounded by a number of water molecules with an oppositely charged pole of solvent water oriented in the direction of the ion concerned.

Ion-dipole and ion-induced dipole forces operate much like dipole-dipole and induced dipole-dipole interactions. However, ion-dipole forces involve ions instead of solely polar molecules. Ion-dipole forces are stronger than dipole interactions because the charge of an ion is much greater than the charge of a dipole.
The strength of ion-dipole interaction depends on the charge and the size of the ion and the magnitude of the dipole moment and the size of the dipole. Ion-dipole bonding is also stronger than hydrogen bonding. An ion-dipole force consists of an ion and a polar molecule aligning so that the positive and negative charges are next to one another, allowing for maximum attraction. Intermolecular ion-dipole forces are much weaker than covalent or ionic bonds.