Science > Chemistry > States of Matter > Change of State of a Substance
In this article, we shall study a change in the state of a substance.
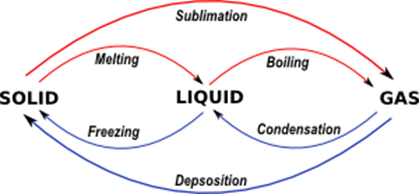
Melting (Solid → Liquid):
The process of change of solid substance into its liquid state is called melting or fusion. The constant temperature at which the solid becomes liquid upon absorption of heat at constant pressure is called the melting point of that solid at that pressure.
Generally melting point increases with the increase in pressure. Ice is the exception to this because its melting point decreases with the increase in the pressure. Melting point at standard pressure is a characteristic property of a substance. The melting point decreases with the addition of the impurity. Hence melting point can be considered as criteria for purity.
Melting points of some important substances are ice (0 °C), iron (1535 °C), aluminium (660 °C), gold (1064 °C), silver (961 °C), aluminium (660 °C), tin (232 °C), zinc (419.5 °C), copper (1084°C), etc.
Explanation on the Basis of Kinetic Model:
When solids are heated the thermal energy of particles increases. Thus the cohesive forces between the particles weaken to such extent that the particles can have relative motion with respect to each other but cannot move out of the bulk. Thus solid gets converted into liquid (melts).
Applications of Melting:
- Melting is very important in the production of alloys. If a binary alloy is to be produced. The element with a higher melting point is melted in a crucible and an element with a lower melting point is added to the molten metal. The second element also melts forming almost a homogeneous solution called alloy. Alloys have many applications in everyday life. Some examples of alloys are
| Alloy | Composition | Applications |
| Babbitt Metal | Sn: 90%, Sb: 7% & Cu: 3% | Used in bearings |
| Bell Metal | Cu: 77% & Sn: 23% | Casting of bells. |
| Brass | Mainly Cu with up to 50% Zn | Imitation jewellery, couplings, utensils |
| Bronze | Mainly Cur with up to 12% Sn | Coins, medals, heavy gears, |
| Duralumin | Al: 95%, Cu: 4%, Mn ‹1%, Mg: 0.5% | Aircraft, boats, railroad cars. |
| Gun Metal | Cu: 85-90%, Sn: 8-12% &Zn: 1-3% | Mainly used for making guns |
| Monel | Ni: 60%, Cu: 33% & Fe: 7% | Corrosion-resistant containers. |
| Phosphor bronze | Bronze with little phosphorus | Springs |
| Solder metal | Pb: 50% & Sn: 50% | Joining two metals to each other. |
- Substances with a high melting point are used to make high-temperature devices. For example, tungsten is used in an incandescent bulb.
- Metals are melted and they are cast (moulded) to give the solids required shape.
Factors Affecting Melting Point:
Internal factors:
- Inter-Molecular (Particle) Forces: If the attractive forces between the molecules of solid are weaker and then the solid has a low melting point. The attraction between the molecules of covalent compounds is weaker than that in ionic solids and hence covalent compounds have a lower melting point than that of the ionic compounds.
- The shape of molecules: If the shape of the molecule is such that it can have a closed packing of the molecules, then the substance has a higher melting point.
- Size of the molecule: The smaller size of molecules can have a closed packing (less void space) of the molecules, then the substance has a higher melting point.
External Factors:
- Impurity: The melting point of a substance decreases with the presence of impurities in it, The phenomenon is called melting point depression. The particles of impurity disrupt the repeating pattern of forces that hold the solid together. Hence less energy is required to melt the part of the solid surrounding the impurity. Salt is spread on the frozen street so that the melting point decreases and the ice melt fast.
- Pressure: For the solids, those expand on heating, the melting point increases with increase in the pressure. It is due to the fact that the pressure opposes the increase in the distance between molecules (expansion). e.g. silver, gold, copper, paraffin wax, etc. For the solids, those contract on heating, the melting point decreases with increase in the pressure. It is due to the fact that the pressure supports the decrease in the distance between molecules (contraction). e.g. ice, cast iron, bismuth, brass, etc.
When two ice cubes are pressed together they form a single block of ice. The phenomenon is called regelation. When the two cubes are pressed against each other. the ice at the interface melts due to lowering of melting point. When the pressure is released the melted ice (water) at the interface solidifies again and a single block of ice is obtained.
Sublimation (Solid ⇔ Gas):
Sublimation is the process by which a heated solid directly changes into its gaseous state i.e. vapour state. These vapours on cooling directly give solid. Such substances are called sublimates. Examples are ammonium chloride, ammonia, naphthalene balls, camphor, etc.
Explanation on the Basis of Kinetic Model:
Certain solids are heated the thermal energy of molecules increases so that the interparticle forces become negligible and the particles can move freely. Thus such solids on heating get converted directly into gases. This phenomenon is known as sublimation. The cohesive forces between the particles in such substances are weak.
Freezing (Liquid → Solid):
The process of change of matter from a liquid state to a solid state is called freezing or solidification. The constant temperature at which a liquid changes into solid by giving out heat energy (or cooling) is called the freezing point of the liquid. The freezing point of a liquid is a characteristic property of the liquid. Hence can be considered as criteria of purity.
Freezing points of some important substances are water (0 °C), benzene (5.5 °C), mercury (- 38.87 °C), etc.
Explanation on the Basis of Kinetic Model:
When liquids are cooled the thermal energy of particles decreases. Thus the cohesive forces between the particles strengthen to such extent that the particles can not have relative motion with each other and they occupy the fixed positions. Thus liquid gets converted into solid (freezes).
Applications of Freezing:
- It is used for the preparation of ice creams.
- The lowering of the freezing point on the addition of solute to the solution is used to find molecular mass of the solute.
Factors Affecting Freezing Point:
For the same substance, the freezing point of the liquid is equal to the melting point of the solid. Therefore the factors those affect melting point of solid obviously affect the freezing point of the liquid.
Freezing Mixtures:
a mixture of two or more substances (e.g. ice water and salt, or dry ice and alcohol) which can be used to produce temperatures below the freezing point of water.
A freezing mixture of 3 parts of ice and 1 part of NaCl produces a temperature of – 21 °C. A freezing mixture of 2 parts of ice and 3 parts of K2CO3 produces a temperature of – 46 °C. A freezing mixture of dry ice and alcohol or ethers can produce a temperature of – 60 °C.
In a freezing mixture, a soluble salt is added. The heat required to dissolve one mole of soluble solute in a solvent is called heat of solvation. This heat required for dissolution of solid is taken from the mixture itself and thus the freezing point decreases in steps.
Freezing mixtures ate used to preserve perishable foodstuff like meat and fishes. They are used for producing sub-zero temperatures in laboratories and industrial units.
Evaporation or Vaporization (Liquid → Gas):
The process of conversion of a substance from the liquid state to its vapour state at any temperature below boiling point is called evaporation or vaporization.
Explanation on the Basis of Kinetic Model:
Some particles from liquid surface possess kinetic energy sufficient to overcome the attractive forces from remaining particles of the liquid and become completely free and escape out as a gas particle in the surroundings. This phenomenon is called evaporation or vaporization.
The rate of evaporation is directly proportional to the surface area and the temperature of the liquid.
During evaporation, the temperature of liquid falls. To maintain temperature balance the liquid particles absorb heat from the surroundings making the surrounding cooler. We have already seen that the molecules with higher kinetic energy leave the surface of the liquid, thus there is an overall decrease in the kinetic energy of liquid. This is one of the reasons for the decrease in the temperature of the liquid.
To increase the rate of evaporation we should increase the surface area, the temperature and the wind speed and should decrease the humidity.
Characteristics of Evaporation:
- It is a surface phenomenon as it takes place on the surface of the liquid.
- It takes place at all temperatures.
- It is a slow process
- The temperature of liquid falls.
Applications of Evaporation:
- During hot day sweat is formed on the body which evaporates. The necessary heat required for the evaporation of the sweat is taken from the body and thus the body temperature is maintained.
- Common salts are produced in shallow lagoons. The water from creek or sea is collected. Water evaporates leaving common salt behind.
- Water gets cooled in an earthen pot (matka). Water seeps through the porous earthen pot and gets on the surface of the pot. It evaporates and the necessary heat required for the evaporation of the water is taken from the water inside the pot and thus the temperature of the water inside the pot decreases.
- Drying of clothes is due to evaporation of water. We have to spread the clothes (increase in surface area), under the sun (increasing temperature) at a windy place.
- In refrigerator the cooling gas (freon) gets evaporator in tubes surrounding freezer region, The necessary heat required for the evaporation of the water is taken from the freezer region and thus the temperature of the freezer region decreases.
Boiling (Liquid → Gas):
Boiling process of change of a liquid into a vapour at a particular temperature and pressure from all part of the liquid. Boiling is a bulk process and takes place throughout the liquid.
When we supply heat energy to liquid the particles start moving faster. At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other. At this temperature, the liquid starts changing into a gas (vapours). The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Pure liquids have fixed boiling points. It can be considered as the criteria of purity.
The constant temperature at which a liquid changes to vapour under normal atmospheric pressure is called the boiling point of the liquid. Boiling points of some important liquids are water (100 °C), Ethyl alcohol (78.3 °C), benzene (80.2 °C), chloroform (62 °C), sulphuric acid (280 °C), diethyl ether (35 °C), etc.
Explanation on the Basis of Kinetic Model:
During boiling, not only the particles on the surface of the liquid but those near walls of the container also start leaving the liquid. It can be seen that small vapour bubbles are formed inside the liquid on walls of the container. As temperature increases the pressure of vapours in bubble increases. The bubbles start growing in size. A point is reached when the vapour pressure inside the bubble is equal to that of atmospheric pressure. At that instant, the bubble detaches from the walls of the container and rise upward. Reaching the surface it bursts giving vapours to the surroundings. Thus there is continuous agitation of the mass of liquid and we say liquid is boiling.
As the pressure increases the boiling point increases. Soluble impurities increase boiling point.
Characteristics of Boiling:
- It is a bulk phenomenon as it takes place throughout the liquid.
- It takes place at fixed temperatures.
- It is a fast process
- The temperature of liquid remains constant.
Factors Affecting Boiling Point:
- Pressure: As the external (atmospheric) pressure decreases boiling point decreases. Hence at higher altitude water boils below 100 °C. Hence higher altitude, food is not cooked properly. To avoid this problem pressure cooker is used for cooking food.
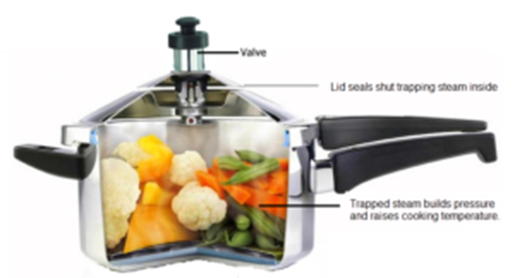
Working of Pressure Cooker:
The basic principle of a pressure cooker is that the boiling point of water increases with the increase in pressure. A pressure cooker is a steel or aluminum vessel with a lid which is airtight. There is a safety valve to release steam to decrease the excess pressure above certain designated pressure. The steam is formed from water in the pressure cooker which has no escape route gets collected in the vessel which put extra pressure on water, which leads to increase in the boiling point of water above 100 °C. Thus gradually the boiling point of water goes on increasing. When the required pressure is reached, the safety valve lifts due to steam pressure and excess of steam is blown out. The safety wall closes and the process restarts. The pressure of steam is even throughout the vessel and hence the food is cooked fast and evenly. The pressure cooker saves a lot of fuel required for cooking.
- Impurity: When a solid is dissolved in liquid the boiling point increases beyond the normal boiling point. Hence during steaming of food, some salt is added to water, so that the food cooks well.
Liquefaction or Condensation:
Liquefaction is the process in which the gaseous substance changes into a liquid state at a particular temperature.
Explanation on the Basis of Kinetic Model:
On cooling the particles of gas lose their kinetic energy and their speed decreases. The decrease in their speed reduces interparticle space and the particles come so close so that the attractive forces between them increase and the gas gets converted into a liquid.