Amino acids contain an amino (–NH2) and carboxyl (–COOH) functional groups. The amino acids in proteins are called alpha (α)-amino acids because the amino group is attached to the α-carbon. A carbon directly connected to carbonyl carbon is termed α-carbon.
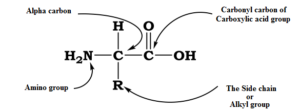
Depending upon the relative position of the amino group with respect to the carboxyl group, the amino acids can be classified as α, β, ϒ, δ, and so on. Only α -amino acids are obtained by the hydrolysis of proteins. They may contain other functional groups also.
All α-amino acids have trivial names, which usually, reflect the property of that compound or its source. Glycine is so named since it has a sweet taste (in Greekglykos means sweet) and tyrosine was first obtained from cheese (in Greek, tyros means cheese.).
Amino acids are generally represented by a three-letter symbol. Sometimes one letter symbol is also used.
The amino acids, which can be synthesised in the body, are known as non-essential amino acids. On the other hand, those which cannot be synthesised in the body and must be obtained through diet, are known as essential amino acids(marked with an asterisk in Table). The deficiency of essential amino acids may cause diseases like Kwashiorkor in which water balance of the body is disturbed.
Characteristics of Amino Acids:
- They are usually colourless, crystalline solids.
- These are water-soluble, high melting solids and behave like salts rather than simple amines or carboxylic acids. This behaviour is due to the presence of both acidic (carboxyl group) and basic (amino group) groups in the same molecule.
- In aqueous solution, the carboxyl group can lose a proton and amino group can accept a proton, giving rise to a dipolar ion known as a zwitterion. This is neutral but contains both positive and negative charges. In zwitterionic form, amino acids show amphoteric behaviour as they react both with acids and bases.
- Except for glycine, all other naturally occurring α-amino-acids are optically active, since the α-carbon atom is asymmetric. These exist both in ‘D’ and ‘L’ forms. Most naturally occurring amino-acids have L-configuration. L-Aminoacids are represented by writing the –NH2 group on the left-hand side.

Classification of Amino Acids:
All amino acids (except proline) contain –H, -NH2, and –COOH bound to the α-carbon.They are differentiated by the side chains (called R-groups or alkyl groups) also bound to the α-carbon. Depending on the nature of alkyl group they are classified into four groups: nonpolar, polar acidic, polar basic, and polar-neutral amino acids.
They are known by common names and each is abbreviated using a three-letter code.
Non-Polar Amino Acids:
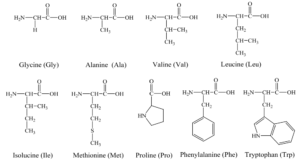
Polar Acidic Amino-Acids:

Polar Basic Amino-Acids:

Polar Neutral Amino-Acids:

Stereo Isomerism in Amino Acids:
Isomers having the same molecular formula, same structural formula but different configurations are called stereo Isomers and the phenomenon is known as stereoisomerism.
Stereoisomers which are related to one another as an object and its non-superimposable mirror image are termed enantiomers. They differ from one another in their behaviour towards the plane polarised light. They are also called enantiomorphs OR optical antipodes. An object which is non-superimposable on its mirror image is said to be a Chiral or dissymmetric object. Optical activity is due to molecular dissymmetry.
An asymmetric carbon atom is defined as carbon atom which has four different monovalent atoms or group attached to its four valencies. It is shown by C*
All α-amino acids, except glycine (because it has two hydrogen atoms attached to the α-carbon), are chiral because the α-carbon is bound to four different groups. As chiral molecules, they can exist as D (dextro) or L (laevo) isomers.

Using convention for writing Fischer projections for an amino acid, the -COOH group is always written at the top and the R group at the bottom. If the NH2 is on the Left we have the L- isomer, if it is on the right, we have the D- isomer. In biological systems, only L isomers are found in proteins
Amino Acids as Acids and Bases:
An amino acid contains the acid group (-COOH) and basic group (-NH2).

The acid group has a tendency of donating proton H+ and the basic group has the capacity to gain the lost proton by the acidic group. Thus a dipolar ion is formed. This dipolar ion is called as a zwitterion (from the German meaning “double ion”).
When the carboxylic acid gives H+ it becomes carboxylate (COO-).For example; glutamic acid (H+ present) and glutamate (H+ absent).

Zwitterions are simultaneously electrically charged and electrically neutral. Zwitterions gain H+ in acidic solutions and lose H+ in basic solutions.

The pH at which the amino-acids exist as zwitterion is called isoelectric point. Different amino acids have different isoelectric points. The isoelectric point of amino-acids depends on the functional group present in the amino-acid. Neutral amino-acids have an isoelectric point in the range of 5.6 to 6.3. At this p[oint they have the least solubility. This property is used to separate different α- amino-acids obtained by hydrolysis of proteins.