Unicellular organisms are capable of having an independent existence and of performing the essential functions (metabolic activities) of life. Anything less than a complete structure of a cell does not ensure independent living. Hence, the cell is the fundamental structural and functional unit of all living organisms. All the living organisms, from the smallest (unicellular) to the largest (multicellular) are composed of cells. To understand metabolic activities of the cell we have to understand the constituent molecules of the cell. The collection of various type of molecules in a cell is called cellular pool. The cellular pool consists of organic and inorganic molecules. The inorganic molecules are water, salts, mineral ions. The organic molecules are carbohydrates, proteins, lipids, nucleic acids etc.
Metabolism:
The sum of the physical and chemical processes in an organism by which its material substance is produced, maintained, and destroyed, and by which energy is made available is called metabolism. These are a variety of reactions carried out at the cell level. The metabolic reactions are divided into two categories
Anabolic Reactions:
- In these reactions biosynthesis of a new cellular material takes place.
- It is the building up of components of cells such as proteins, nucleic acids.
- In These reactions, energy is consumed.
Catabolic Reactions:
- In these reactions complex, stored substances are hydrolysed and broken down into simpler molecules.
- It is breaking down of components of a cell such as breaking of glucose into pyruvates.
- In these reactions, energy is produced.
Both the reactions require bio-catalyst commonly known as enzymes.
Water:
The chemical reactions of all living things take place in an aqueous environment. Water has several properties that make it one of the most important compounds found in living things
Polarity:

A water molecule, H2O, is covalent compound formed by sharing electrons by the hydrogen and oxygen atoms. but these two atoms do not share the electrons equally. Oxygen being more electronegative pull the shared pair of electrons towards itself. In this process acquires partial negative charge and hydrogen acquire a partial positive charge. As a result, the electrical charge is unevenly distributed in the water molecule.
Although the total electrical charge on a water molecule is zero, the region of the molecule where the oxygen atom is located has a slight negative charge (2δ–), while the regions of the molecule where each of the two hydrogen atoms is located each have a slight positive charge (δ+).
Due to this uneven pattern of charge, water is a polar molecule. This polar nature of water makes it a good solvent. An ionic compound dissolved in water tends to dissociate into ions. This breaking up of an ionic compound means the ions can participate in many biological reactions.
Hydrogen Bonding:
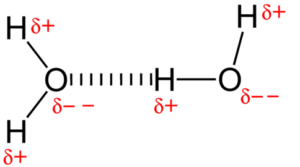
Due to polar nature of water, its molecules attract one another or stick together. This attraction between water molecules is caused byhydrogen bonding. A positive region of one water molecule is attracted to the negative region of another water molecule. Hydrogen bonds are weak bonds and can be easily broken
The hydrogen bonds in water exert a significant attractive force, causing water to attach or hold on to itself (Cohesion) and to other surfaces (Adhesion). This force of adhesion and cohesion enable water molecules to move upwards through narrow tubes against the force of gravity. This phenomenon is known as a capillarity. Water moves up a plant stem through cohesion-tension in the xylem due to the hydrogen bonds.
Homeostasis:
Homeostasis can be defined as a property of an organism or system that helps it maintain its parameters within a normal range of values. It is key to life, and failures in homeostasis can lead to diseases.
Water must gain or lose a large amount of energy for its temperature to change, thus it makes it a stable environment to live in. Water has large specific heat hence it has the ability to absorb large amounts of energy helps to keep cells at an even temperature despite changes to the external temperature.
Carbohydrates:
- Carbohydrates are polyhydroxy aldehydes or polyhydroxy ketones or compounds that give polyhydroxy aldehydes or polyhydroxy ketones on hydrolysis.
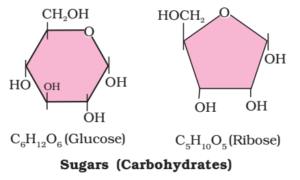
- They contain at least one asymmetric carbon atom. e.g. Glucose (C6H12O6), Cane sugar Sucrose (C12H22O11).
- They are a class of naturally occurring organic compounds found in plants. These are the organic compounds produced in the chlorophyll containing cells during photosynthesis.
- They contain carbon, hydrogen, and oxygen actually carbohydrates means hydrates of carbon. The general proportion of hydrogen and oxygen is definite 2:1.
- Simple carbohydrates are commonly known as sugars (glucose, fructose). They take part in the metabolic reaction. While complex carbohydrates like starch and cellulose etc form storage and structural units.
- The cells of the human body obtain most of their energy from carbohydrates.
Classification of Carbohydrates:
Classification Based on Solubility and the Taste:
the carbohydrates are classified into two types. a) Sugars and b) Nonsugars.
Characteristics of sugars:
- They are soluble in water.
- They are crystalline in nature.
- They are sweet in taste.
- e.g. Cane sugar, Glucose etc.
Characteristics of non-sugars:
- They are insoluble in water.
- They are amorphous in nature.
- They are tasteless
- e.g. Starch, cellulose etc.
Classification Based on Hydrolysis Behaviour the Carbohydrates:
On the basis of hydrolysis behaviour carbohydrates are classified into three types. a) Mono-Saccharides b) Oligo-Saccharides and c) Poly-Saccharides.
Monosaccharides:
Carbohydrates which are basic units or which can not be hydrolyzed further are called monosaccharides.
Characteristics of monosaccharides :
- They are basic units of carbohydrates.
- They can not be hydrolysed further into small units.
- They contain six carbon atoms in a molecule
- They are water soluble and sweet in taste.
- Depending upon number of carbons they are further classified as Triose, n=3 (glyceraldehyde, dihydroxyacetone) Pentose, n=5 (ribose/ deoxyribose) Hexose, n=6 (glucose/ fructose / galactose)
- Depending upon the presence of an aldehydic group or a ketonic group they are further sub classified into aldoses and ketoses respectively.
Aldoses: The monosaccharides containing aldehydic group are called aldoses. Examples: Aldopentose C5H10O5 – Arbaniose, Xylose, Ribose. Aldohexose C6H12O6 – Glucose, Galactose
Ketoses: The monosaccharides containing ketonic group are called ketoses. Examples: Ketopentose C5H10O5 – Ribulose.Ketohexose C6H12O6 – Fructose.

Oligosaccharides:
Carbohydrates which on hydrolysis give definite no. of monosaccharides i.e. 2 to 9 molecules of monosaccharides are called oligosaccharides.
Depending upon no. of monosaccharides formed on hydrolysis they are further sub classified into Di-saccharides, Tri-saccharides, Tetra-saccharides etc.
Di-saccharides:
Di-saccharides on hydrolysis give two molecules of monosaccharide. The covalent bond that joins two monosaccharides is called the glycosidic band.
e.g. Cane sugar (Sucrose) (C12H22O11)on hydrolysis gives one molecule of glucose and one molecule of fructose. Maltose (C12H22O11) on hydrolysis gives two molecules of glucose, Lactose (C12H22O11) on hydrolysis gives one molecule of glucose and one molecule of galactose.
Disaccharides are crystalline, water soluble and sweet in taste. They have general formula (C12H22O11)

Tri-saccharides:
Tri-saccharides on hydrolysis give three molecules of monosaccharide. e.g. Raffinose (C18H32O16).
Tetra-saccharides:
Tetra-saccharides on hydrolysis give three molecules of monosaccharide. e.g. Stachyose (C24H42O21).
Polysaccharides:
- Carbohydrates which on hydrolysis give indefinite or large no. of monosaccharides (more than 10) are called polysaccharides.
- They are natural polymeric carbohydrates.
- They are insoluble in water, amorphous and tasteless. They are nonsugars.
- They have general formula (C6H10O5)n. e.g. Starch, Cellulose, Inulin, Dextrin etc.Cellulose is a linear polymer of β-Glucose units while starch is a branched polymer of α-glucose units.
Sucrose → Glucose + Fructose
Maltose → Glucose + Glucose
Lactose → Glucose + Galactose
Raffinose → Glucose + Fructose + Galactose
tachyose → Glucose + Fructose + Galactose + Galactose
The role of Carbohydrates:
- It provides energy for metabolism.
- The monosaccharides glucose is required for the production of ATP.
- In mammals disaccharide lactose present in milk provides energy to their bodies.
- The polysaccharides like cellulose serve as a structural member of the cell membrane and cell wall.
- The polysaccharide like starch serves as stored food.