Science > Biology > Gene its Nature, Expression and Regulation > Nucleotides
In this article, we shall study structural units of nucleic acid called nucleotides.
In 1869, Friedrich Miescher separated cellular substance from the nuclei of pus cell and called it ‘Nuclein’. Due to acidic nature, the substance is further called as nucleic acid. There are two types of nucleic acids a) Deoxyribonucleic acid (DNA) found primarily in the nucleus of cells and b) Ribonucleic acid (RNA) found mainly in the cytoplasm of living cells.
Chemical Components of Nucleic Acids:
Nucleotides:
Nucleotides are the structural units of nucleic acids. Each nucleotide has three components
Sugars:
The five-carbon sugar (pentose) in nucleic acids is ribose or a ribose derivative. It has a pentagonal ring structure. In RNA the sugar is ribose, in DNA it is 2-deoxyribose.
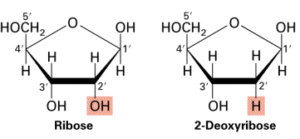
The only difference between these two sugars is found at the 2-carbon of the ribose ring. Ribose has a hydroxyl group (-OH) bound to this carbon, while deoxyribose has a hydrogen atom (“deoxy” means no oxygen).
Phosphate Group:
The second component of a nucleotide is derived from phosphoric acid (H3PO4).
Phosphoric acid contains three hydroxyl groups attached to phosphorous.
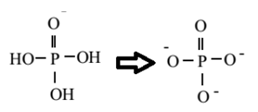
Phosphoric acid Phosphate group
From these three OH groups, two are responsible for strand formation.
Nitrogen or Organic Bases:
The organic bases found in nucleic acids are derivatives of pyrimidine or purine.
Pyrimidine is a six-membered heterocyclic ring. A heterocyclic ring is a ring compound containing atoms that are not all identical. Purine is a fused ring compound containing a six-membered ring connected to a five-membered ring.
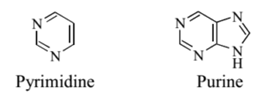
Pyrimidines:
There is only one ring which is hexagonal and heterocyclic. The ring consists of four carbons and three nitrogens with an alternate single and double bond. Numbering is done clockwise starting from nitrogen. Nitrogen atoms are present at the first and third positions. Rest positions are occupied by carbon. Such a ring is called a pyrimidine ring.

The three pyrimidine derivatives found in nucleic acids are cytosine (C), thymine (T), and uracil (U).

Cytosine = 2-oxy-4-amino pyrimidine
Thymine = 2,4-dioxy-5-methyl pyrimidine
Uracil = 2,4-dioxy pyrimidine
Characteristics of Pyrimidines:
- They are single ring compounds.
- They are formed by a pyrimidine ring.
- There are 4 carbons and 2 nitrogens in the ring.
- Nitrogen atoms are present at the first and the third position.
- Oxygen is attached to second carbon by a double bond.
- A glycosidic bond is formed between nitrogen at the first position in pyrimidine and carbon at the first position in pentose sugar.
Purines:
There are two rings (dicyclic) in this nitrogen compound. There are nine atoms in the molecule of which 4 are nitrogen and 5 are carbon atoms. There are 6 atoms in the first ring called pyrimidine ring and 5 atoms in the second ring called imidazole ring. Atoms are numbered anticlockwise in pyrimidine ring and clockwise in the imidazole ring. The imidazole ring.is fused with pyrimidine ring at the 4th and 5th position so that the two rings share carbon atom at 4th and 5th position. The nitrogen is present at first, third, seventh and ninth position in the ring.

The two purine derivatives found in nucleic acids are adenine (A) and guanine (G).

Characteristics of Purines:
- They are double ring compounds.
- They are formed by pyrimidine and imidazole ring.
- There are 5 carbons and 4 nitrogens in the ring.
- Nitrogen atoms are present at the first, third, seventh and ninth position.
- No oxygen is attached to the second carbon.
- A glycosidic bond is formed between nitrogen at the ninth position in pyrimidine and carbon at the first position in pentose sugar.
Note:
- Adenine, guanine, and cytosine are found in both DNA and RNA. Thymine is found only in DNA, while uracil is found only in RNA.
- Thymine and uracil are often used to differentiate DNA from RNA.
Nucleosides:
When ribose or 2-deoxyribose is combined with a purine or pyrimidine base, then the combination is called nucleoside. A nucleoside is basically a nucleotide that is missing the phosphate portion.
Thus Nucleoside = Sugar + Nitrogen Base
In a nucleoside, the pentose sugar and base are joined by an N-glycosidic bond formed between semialdehyde -OH group of monosaccharide at 1 and H of the pyrimidine base at N-1 or the purine base at the 9th nitrogen atom of the ring

New Naming System for Nucleosides:
|
Base |
Nucleioside |
|
|
Ribose
in RNA |
Adenine |
Adenosine |
|
Guanine |
Guanosine |
|
|
Cytosine |
Cytidine |
|
|
Uracil |
Uridine |
|
|
Deoxyribose
in DNA |
Adenine |
Deoxyadenosine |
|
Guanine |
Deoxyguanosine |
|
|
Cytosine |
Deoxycitidine |
|
|
Thymine |
Deoxythimidine |
Nucleotides:
The nucleotides are named according to their nitrogenous base. For e.g. a nucleotide containing thymine is called thymine nucleotide.
Thus Nucleotide = Pentose Sugar + Nitrogen Base + Phosphate Group
or Nucleotide = Nucleoside + Phosphate Group
New Naming System for Nucleotides:
|
Base |
Nucleioside |
Nucleotides |
|
|
RNA |
Adenine |
Adenosine |
Adenosine-5’-monophosphate (AMP) |
|
Guanine |
Guanosine |
Guanosine-5’-monophosphate (GMP) |
|
|
Cytosine |
Cytidine |
Cytidine-5’-monophosphate (CMP) |
|
|
Uracil |
Uridine |
Uridine-5’-monophosphate (UMP) |
|
|
DNA |
Adenine |
Deoxyadenosine |
Deoxyadenosine-5’-monophosphate (dAMP) |
|
Guanine |
Deoxyguanosine |
Deoxyguanosine-5’-monophosphate (dGMP) |
|
|
Cytosine |
Deoxycitidine |
Deoxycitidine-5’-monophosphate (dCMP) |
|
|
Thymine |
Deoxythimidine |
Deoxythimidine-5’-monophosphate (dTMP) |
Linking of Nucleotides in Polynucleotides:
A polynucleotide chain is formed by connecting several nucleotides in succession. Several thousand nucleotides are linked together by 3′-5′ phosphodiester bond in which the phosphate group carried in 5th carbon atom of pentose in one nucleotide is linked to 3′ hydroxyl group of 3′ carbon of the pentose of the next nucleotide. These bonds provide considerable stiffness to polynucleotide chain.

The bond is called phosphodiester bond because one molecule of phosphoric acid joins with sugar molecules of two nucleotides through an ester linkage.
Joining two nucleotides is called dinucleotide, joining three nucleotides is called trinucleotide and so on. A chain up to joining of twenty nucleotides is called oligonucleotide. If there is joining of more than twenty nucleotides it is called polynucleotide.
RNA is a polynucleotide that, upon hydrolysis, yields D-ribose, phosphoric acid, and the four bases adenine, guanine, cytosine, and uracil.
DNA is a polynucleotide that yields D-2′-deoxyribose, phosphoric acid, and the four bases adenine, guanine, cytosine, and thymine.
The Directionality of Polynucleotide Chain:

Adjacent nucleotides in a single strand of the polynucleotide are joined by a phosphodiester bond between their 3′ and 5′ carbons. This means that the respective 5′ and 3′ carbons are exposed at either end of the polynucleotide, which are therefore called the 5′-P end and the 3′-OH end. These are also called the phosphoryl (5′-P terminus) and hydroxyl (3′-OH terminus) ends, respectively, because of the chemical groups typically found at those ends.
One reply on “Nucleotides”
Essential aspect is fully described in simple and easy way❤️