In this article, we shall study the initial classification of elements and Mendeleev’s periodic table.
Need for Classification of Elements:
With the rapid advance and developments in science, the number of discovered elements increased. Due to a large number of elements, it is difficult to study and remember the behaviour and properties of each and every element. Hence attempts have been made to classify these elements into groups of elements having similar characteristics. This method of grouping the elements into different classes is known as periodic calcification classification of elements. This classification of elements makes the study of elements systematic and easy.
Unitary Theory of Periodic Classification:
This theory was proposed by William Prout in 1815. He suggested that the values of atomic masses of all elements were whole numbers or varied only slightly from the whole numbers if hydrogen was considered the basis of all atomic masses. Thus hydrogen was regarded as the ‘central or base’ element around which all other atoms were made. Thus 12C (carbon) is made up of 12 units of hydrogen. 20Ca (calcium) is made up of 20 units of hydrogen.
This theory was not able to explain fractional atomic masses of some elements 63.5Cu (copper) and 35.5Cl (chlorine), etc.
Dobereiner’s Triads:
This theory was given by a German chemist Dobereiner. According to the theory, If three elements resembling properties are arranged in increasing atomic masses, then in each case, the atomic weight of the middle element is found to be the arithmetic mean (average) of the other two extreme elements. This relation is sometimes referred as the law of triads. The group of such three elements is called a triad.
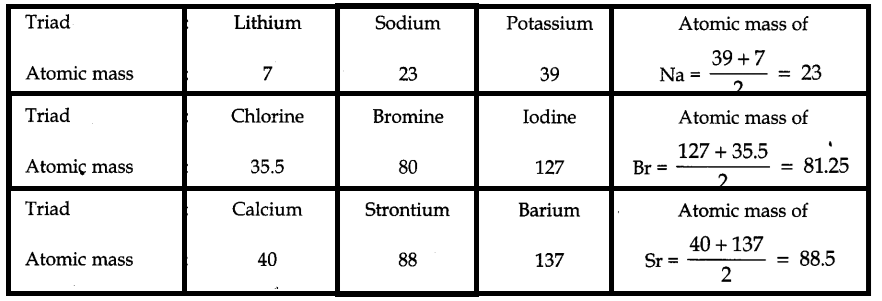
However, this theory suffered a major limitation as Dobereiner could classify only 9 elements in such a manner, out of all those were known at the time. Therefore, the concept of triads is discarded.
Cooke’s Homologous Series:
In chemistry, a homologous series of elements is a series of elements arranged in ascending atomic mass such that the atomic mass increases in a regular fashion. Cooke classified elements in several homologous series.
| Element | Atomic Mass | Distribution | Type of Atomic Mass |
| Nitrogen | 14 | 14 | a |
| Phosphorous | 31 | 14 + 17 | a + b |
| Arsenic | 75 | 14 + 17 + 44 | a + b + c |
| Antimony | 119 | 14 + 17 + 2 x 44 | a + b + 2c |
| Bismuth | 207 | 14 + 17 + 4 x 44 | a + b + 4c |
Newland’s Law of Octaves:
“Octo” means “eight”. In music, every eighth note is similar to that of the first note and of frequency double the frequency of first. These are seven musical notes according to Indian system, these musical notes are sa, re, ga, ma, pa da, ni and again sa. This sa is similar to the first sa. According to the Western system, the corresponding symbols of these notes are do, re, me, fa, so, la, ti again do. This do is similar to first do. In the musical notes, there is a repetition of the same note after a gap of seven. Thus every eighth note is the repetition of the first note.
In1865, an English chemist, John Alexander Newlands’s observed that when the lighter elements were arranged in order of their increasing atomic masses, the properties of every 8th element were similar to those of the first one like the eighth note of a musical scale. This relation is called Newlands’s law of octaves.
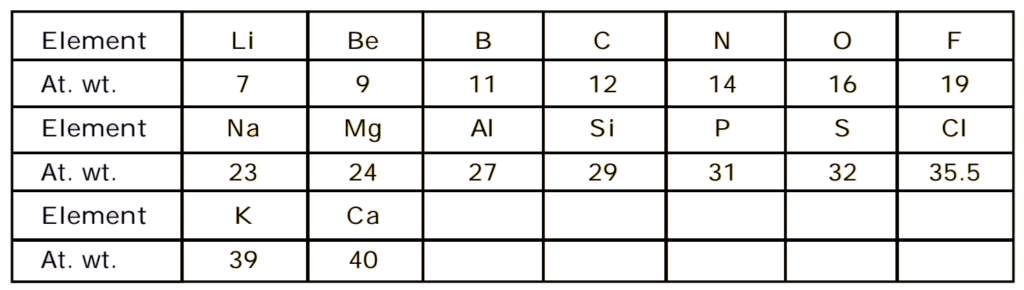
Drawbacks of this concept:
- It was applicable to only lighter elements having atomic weights up to 40 u (calcium). After that, every eighth element did not possess the same properties as by the element lying above it in the same group.
- With the discovery of noble gases, the properties of the 8th element were no longer similar to those of the first one.
Lother Meyer’s Arrangement of Elements:
In 1869, Lothar Meyer, a German chemist, studied the physical properties of the various elements. He Plotted a graph by taking the atomic volume of elements on the y-axis and atomic masses of the elements on the x-axis and observed that the elements with similar properties occupied a similar position on the curve.
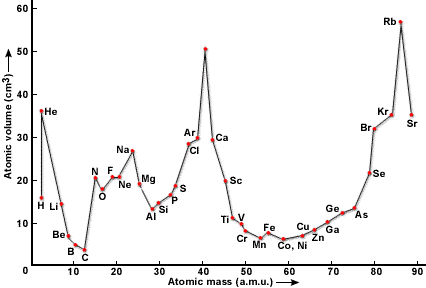
Lothar Meyer proposed that the physical properties of the elements are a periodic function of the atomic masses. He arranged the elements in the tabular form in order of their increasing atomic weights.
Characteristics of Graph:
- The elements with similar properties occupied a similar position on the curve.
- The most strongly electropositive alkali metals occupy the peaks on the curve.
- The less strongly electropositive alkaline earth metals occupy the descending position on the curve.
- The most electronegative elements i.e. halogens occupy the ascending position on the curve.
Mendeleev’s Periodic Table:
The Russian Chemist Dmitri Ivanovich Mendeleev tried to co-relate the atomic masses of the elements with their physical and chemical properties. He used the basis of Lother Meyer’s hypothesis. He studied the formulae and chemical properties of several elements compared to Lother Meyer. On basis of his study, he proposed the Periodic Law.
Mendeleev’s Periodic Law:
It states that the physical and chemical properties of the elements are a periodic function of their atomic masses.
Mendeleev’s Periodic Table:
Mendeleev arranged the elements known at that time in order of increasing atomic masses in horizontal rows and vertical columns. This arrangement was called the periodic table. Elements with similar characteristics were found to present in vertical rows called groups. The horizontal rows were known as periods.
Characteristics of the Periodic Table:
- In the Mendeleev’s periodic table, the elements are arranged in vertical columns called groups and horizontal rows known as periods.
- There are eight groups indicated by Roman Numerals as I, II, III, IV, V, VI, VII, VIII and the elements belonging to the first seven groups have been divided into sub-groups designated as A and B on the basis of similarities in properties. Group VIII consists of nine elements which are arranged in three triads.
- There are six periods (numbered from 1 to 6). The periods 4, 5 and 6 are divided into two halves. The first half of the elements are placed in the upper left corners and the second half occupy lower right corners in each box.
- Mendeleev left some empty spaces in the periodic table for the undiscovered elements which were identified later on and were placed at their respective positions.
- Noble gases are placed in a separate group called a zero group without disturbing the main periodic table.
Merits of Mendeleev’s Periodic Table:
A Systematic study of the elements:
- Mendeleev for the first time arranged a very large number of elements into groups and periods. This made the study of the elements quite systematic.
Prediction of new elements and their properties:
- Mendeleev laid more stress on similarity in properties rather on increasing atomic masses of the elements. So whenever a particular element did not fit in the arrangement, he left a gap in the periodic table. Thus, many gaps for the undiscovered elements were left in the periodic table by Mendeleev.
- He left the gap one place down aluminium and silicon and called these elements as Eka-aluminum and Eka-Silicon. He predicted their physical properties. These elements were discovered afterword and called Gallium and Germanium respectively.
Correction of doubtful atomic masses:
- Mendeleev also corrected the atomic masses of certain elements with the help of their expected positions and properties.
Demerits of the Mendeleev’s Periodic Table:
Position of hydrogen:
- Both hydrogen (H2O, HCl, H2S) and alkali metals like Sodium form similar compounds (Na2O, NaCl, Na2S) with elements like oxygen, chlorine and sulphur etc., hence Hydrogen (Z = 1) is placed at the top of the alkali metal family because it resembles alkali metals in its properties.
- At the same time, hydrogen (CH4, SiH4, GeH4) also resembles halogens present in group VII A like chlorine (CCl4 , SiCl4, GeCl4) in many of its properties. Both are non-metals and also diatomic in nature. The compounds of both hydrogen and halogens with certain non-metals are of covalent nature.
- Hence there was confusion about the position of hydrogen.
Anomalous positions of some elements:
- The elements in the Mendeleev’s Periodic table have been arranged in order of increasing atomic masses, but in some cases, the element with higher atomic mass precedes the element with lower atomic mass. For example, Ar (Atomic mass =39.9) precedes K (Atomic mass= 39.1) and similarly, Co (Atomic mass 58.9) has been placed ahead of Ni (Atomic mass=58.7).
- No justification has been given by Mendeleev for such anomalous positions of these elements.
Position of isotopes:
- The element whose atoms have different atomic masses but the same atomic number are called isotopes. Since the periodic table has been prepared on the basis of increasing atomic masses of the elements, different positions must have been allotted to all the isotopes of a particular element.
- For example, Hydrogen has three isotopes having atomic masses 1, 2, and 3. Hence three different positions should have been allotted to the isotopes of hydrogen. But they have been assigned only one position.
No co-relation of elements in sub-groups:
- According to Mendeleev, the elements placed in the same group must show similar properties. But there is no similarity among the elements in the two sub-groups of a particular group.
- For example, Li, Na, and K present in group IA are quite different from Cu, Ag, and Au which belong to group IB.
Different groups for similar elements:
- According to Mendeleev, the elements having similar properties are placed in the same group of the periodic table. But elements with similar properties have been placed in different groups.
- For example, Both copper and mercury resemble in many properties. But copper has been placed in group IB while mercury has been assigned a position in group IIB
Cause of periodicity:
No proper explanation has been given by Mendeleev as to why the properties of the elements get repeated after gaps of atomic masses in a particular group.