The gaseous compound formed when dry fluorine gas reacts with dry Hydrogen, gas is called hydrogen fluoride. Hydrogen fluoride gas on condensation forms liquid hydrogen fluoride which is also referred to as anhydrous hydrogen fluoride or anhydrous hydrofluoric acid. When vapours of hydrogen fluoride are dissolved in water, the resulting aqueous solution is known as hydrofluoric acid.
Anhydrous HF:
- It is a gaseous compound or dry liquid obtained by condensation of gaseous dry HF.
- Non-conductor of electricity as anhydrous hydrofluoric acid is non-ionised.
- HF stored in steel cylinders
Preparation:
Dry H2 gas + Dry F2 gas and From Fremy’s salt KHF2
Hydrofluoric Acid:
- It Is an aqueous solution of hydrogen fluoride.
- It is a weak monobasic acid. It is a weak electrolyte
- Stored in ‘gutta-percha’ or Polythene bottles.
Preparation:
By action of H2SO4 on CaF2 (Fluorspar):
When powdered fluorspar i.e. CaF2 is mixed with 96% H2SO4 and distilled in lead retort between 473 K to 573 K, vapours of HF are produced.
The vapours of HF are absorbed in water in lead receiver which is kept cooled by immersing it in cold running water. This gives aqueous hydrofluoric acid.
Reaction:
CaF2 + H2SO4 → CaSO4 + 2HF
By Direct Combination of Hydrogen and Fluorine:
Anhydrous HF can be prepared by direct combination of dry hydrogen gas and dry fluorine gas. This reaction occurs even in dark and even at low temperature as low as 63 K
H2 + F2 → 2HF
From Fremy’s salt – Potassium hydrogen fluoride KHF2:
Pure and perfectly dried Fremy’s salt KHF2 is distilled In a copper retort to about 573 K.
KHF2 → KF + HF
The vapours are collected in a copper vessel cooled by a freezing mixture. Anhydrous liquid HF is thus obtained. Copper is not affected by anhydrous-liquid HF. (KF remains in the distilling vessel)
Purification:
- HF is dehydrated using thionyl chloride.
HF + H2O + SOCl2 → HF + 2 HCI + SO2
- On cooling gases, only hydrogen fluoride condenses to a liquid.
- Hydrogen chloride is insoluble in liquid hydrogen fluoride. Sulphur dioxide is removed by fractional distillation.
- Anhydrous HF or aqueous HF are purified by redistillation or fractional distillation.
Storage:
- Both -hydrogen fluoride and hydrofluoric acid attack glass, so they cannot be stored in a glass container.
- Anhydrous hydrogen fluoride can be stored in special steel cylinders.
- Aqueous hydrofluoric acid is stored in ‘gutta-percha’ or Polythene bottles.
Properties of HF:
HF differ from the other halogen hydracids:
- HF is a liquid (B.P. 292.5 K) while the other hydracids are gases.
- The liquid contains associated (HF)n molecules formed due to intermolecular hydrogen bonding. In other hydrogen halides, intermolecular hydrogen bonding does not exist.
- Hydrofluoric acid is the weakest of all the halogen acids.
- It forms acid salts like KHF2.
- HF attacks the glass and dissolves it. Other hydracids do not react with the glass.
- It is the only acid which reacts with Silica (SiO2).
Physical Properties of HF:
- Anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. Boiling point 292.5 K (19.5°C)
- Both the liquid and the vapours are corrosive and poisonous. They attack the skin causing painful blisters.
- The liquid contains associated (HF)n molecules formed due to Inter-molecular hydrogen bonding.
- It dissolves in water in all proportions.
Hydrogen Bonding in Hydrogen Fluoride:
In hydrogen fluoride, the hydrogen atom is covalently bonded to the fluorine atom which is highly electronegative. There is a wide difference in the electronegativities between hydrogen (2.1) and fluorine (4.0). The covalent bond is therefore highly polar and has a partial ionic character. The hydrogen atom in HF has a high partial positive charge and fluorine has a high partial negative charge.
H+δ − F-δ
Negatively charged F atom from one HF molecule attracts the positively charged H atom from the other molecule through electrostatic attraction. This is called Hydrogen bonding. This causes a large number of H-F molecules to associate together. The associated molecules form zigzag chains. More amount of energy is thus required to separate these molecules from each other.
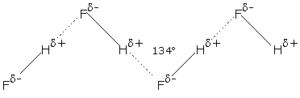
Such strong hydrogen bonding is not possible in the case of other hydrogen halides since other halogens are less electronegative than fluorine.
Although HCI, HBr and HI molecules are polar, the magnitude of polarity is not sufficient to form hydrogen bonds between their molecules. Hence, these molecules exist as gases.
Characteristic Properties of HF due to Intermolecular Hydrogen Bonding:
Hydrogen fluoride is liquid with B.P. 292.5 K, whereas other hydrogen halides are gases. HCI, HBr and HI have B.P. 188 K, 206 K and 238 K respectively. Thus expected B.P. for HF is below 188 K. But it is higher. Thus the unexpectedly higher boiling point of HF is due to intermolecular hydrogen bonding.
Hydrofluoric acid is the weakest of all halogen acids. The degree of dissociation for 0.1 M solutions of HF, HCI, HBr, and HI are 0.08, 0.93, 0.94, and 0.95 respectively at 298 K.
It forms hydrogen-bonded anion like HF2 – and acid salt like KHF2.
HF is a liquid, while other halogen acids are gases at ordinary temperature:
Due to intermolecular hydrogen bonding, HF molecules are associated as (HF)n. Formation of hydrogen bonding lowers energy of the system and so HF is liquid. Such hydrogen bonding is absent in other hydrogen halides. Hence they are gases at ordinary temperature.
Due to hydrogen bonding, the separation of HF molecules requires more energy. Hence the boiling point of HF will be higher than other hydrogen halides, which do not form hydrogen bonding.
HF is the weakest amongst all hydracids of halogens:
Hydrofluoric acid is a weak monobasic acid having Ka = 6.8 x 10-4. The degree of dissociation for 0.1 M solution of HF, HCI, HBr, and Hi are 0.08, 0.93, 0.94, and 0.95 respectively at 298 K. Fluorine has smallest atomic size, hence in H-F molecule, H-F bond length is short.
The highest electronegativity of fluoride makes the H-F bond highly polar. These two factors make the H-F bond strong and therefore, it needs more energy for ionization. Because of intermolecular hydrogen bonding, HF molecules are associated. More energy is required to separate HF molecules for ionization.
Other halogens are larger in size and less electronegative than fluorine. Their halogen acid molecules are not associated. Therefore, HCI, HBr and HI are almost completely ionized in aqueous solution.
Chemical Reactions of Hydrogen Fluoride:
Action of HF on Silica:
A concentrated solution of hydrofluoric acid reacts with Silica (Quartz) to form Silicon tetrafluoride. But in presence of excess of hydrofluoric acid, hydrofluosilicic acid is formed. It is soluble in water.
SiO2 + 4HF → SiF4 + 2 H2O
SiF4 + 2HF → H2 Si F6 (hydrofluosilicic acid)
Action of HF on a Glass:
Ordinary glass is essentially a mixture of the silicates of alkali metals, alkaline earth metals and some free Silica. Hydrogen fluoride attacks glass and decomposes it. Hydrofluosilicic acid and metal silicofluoride are formed which are soluble in water. Therefore, glass is slowly eaten up by HF. Thus glass slowly dissolves in HF acid. Hence HF solution is not stored In glass bottles.
Na2SiO3 + 6 HF → Na2 Si F6 + 3H2O
CaSiO3 + 6 HF → CaSi F6 + 3H2O
SiO2 + 6HF → H2 Si F6 + 2H2O
Uses of HF:
Hydrogen fluoride is used for
- Etching of glass.
- Removal of silica (sand) from graphite.
- Silicate analysis i.e. estimation of silica.
- Preventing dental decay in the form of fluoride ion.
- Liquid HF is used as a non-aqueous solvent.
Etching of a Glass:
The process of making a permanent marking on the glass surface is called etching of the glass. The etching of glass is carried out by treating it with HF (hydrogen fluoride). The glass is a homogeneous mixture of silicates of sodium and calcium along with some free silica. HF reacts with glass-forming metallic fluorides which are water-soluble. Due to this reaction, the glass gets etched.
The reactions involved in the etching of the glass are as follows.
Na2SiO3 + 6 HF → Na2 Si F6 + 3H2O
CaSiO3 + 6 HF → CaSi F6 + 3H2O
SiO2 + 6HF → H2 Si F6 + 2H2O
Process of etching:
- The glass which is to be etched is cleaned with chromic acid and washed with water.
- Then the glass is coated with thin layer of wax.
- Then the markings or designs are engraved on this coated surface with the help of sharp pointer. Thus the wax in the region is removed and the area to be etched is exposed.
- The exposed surface is then treated with aqueous HF solution or HF vapours.
- The chemical reaction takes place and the glass gets etched in the exposed region.
- The rest of the wax is removed by dissolving it in turpentine.
Use of HF in the Removal of Silica from Graphite:
Graphite is used in preparing electrodes in electrolytic cells. In place of natural graphite, artificial graphite is used more commonly. Artificial graphite is prepared by heating coke powder with silica in an electric arc furnace. So artificial graphite contains silica as the impurity. The presence of Silica will increase its resistance. To remove silica, graphite is treated with hydrofluoric acid.
SiO2 + 4HF → SiF4 + 2 H2O
SiF4 + 2HF → H2 Si F6 (hydrofluosilicic acid)