In this article, we shall study halogen member fluorine. Fluorine is positioned in Group 17 and the second period of the periodic table. It is highly reactive.
Occurrence:
Because of extreme reactivity fluorine does not occur in the free state. It occurs as the fluorides (F–) of certain metals such as Calcium and Aluminium. Its major minerals are
- Fluorspar – CaF2,
- Cryolite – Na3AlF6 and
- Fluorapatite -3Ca(PO4)2 , CaF2.
Difficulties in the Isolation of Fluorine:
The following difficulties were involved in the isolation of fluorine.
- Its oxidation is not possible. It exists as fluoride (F–) in its minerals. The preparation of it from a fluoride is an oxidation reaction.
2 F– → F2 + 2e–
- It is the most electronegative element and itself is a very strong oxidizing agent. F2 is a more powerful oxidizing agent than O2. Therefore oxidation of fluoride to fluorine could not be carried out by chemical oxidizing agents. Therefore, no oxidizing agent was able to separate fluorine from hydrogen.
- When hydrofluoric acid is heated with oxidizing agents, like MnO2, KmnO4, K2Cr2O7 no fluorine is obtained. This is because of the High affinity of fluorine for hydrogen.
- H-F bond Is considerably strong due to small bond length and high polarity. It cannot be prepared by the electrolysis of HF. Electrolysis of hydrofluoric acid solution gave ozonized oxygen at the anode, instead of fluorine. This is due to the action of F2 on water.
2H2O + 2F2 → 4HF + O2
3H2O + 3F2 → 6HF + O3
- Then the electrolysis of anhydrous hydrogen fluoride was attempted. It was found to be a poor conductor (non-conductor) of electricity. HF is highly volatile and poisonous.
- Proper apparatus for preparation and storage was not available. As F2 is extremely reactive, it attacks glass, platinum, carbon, and other materials commonly used for the construction of the apparatus of electrolysis.
- Aqueous HF is an active solvent. It attacks the glass and various metals. Thus the main need was to find a suitable apparatus end suitable electrolyte.
Preparation of Fluorine:
Dennis Method:
Principle:
Pure, dry and anhydrous potassium hydrogen fluoride (KHF2) is electrolyzed in the molten state at 523 K when hydrogen is liberated at the cathode while F2 is liberated at the anode.
Diagram:
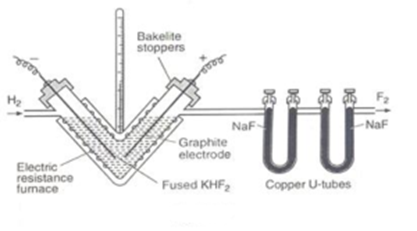
Dennis Cell (Apparatus):
This is a heavy V-shaped copper tube. F2 reacts with copper forming a protective layer of copper fluoride CuF2. This protects the cell from further attack by fluorine. The V-shaped tube prevents fluorine liberated at the anode and hydrogen at the cathode from coming in contact with each other.
Electrodes: The electrodes are made of graphite. The copper tube is closed with copper caps through which graphite electrodes are fitted. Copper caps are cemented to and Insulated from the copper tube by bakelite cement.
Electrolyte: Pure, dry and anhydrous KHF2, in a molten condition. Anhydrous HF Is added periodically.
Temperature: 523 K
Heating unit: The copper tube is covered from outside, with asbestos cement insulator of current but conductor of heat resistance wire for electrical heating legging – to prevent loss of heat by radiation.
Outlets: Two outlets are provided for hydrogen and fluorine in the upper portions of the two arms.
Current and Voltage: A current of 5 amperes and at 12 volts is employed.
Reactions:
2KHF2 → 2KF + 2HF
2KF → 2K+ + 2 F–
(HF acts as an ionizing liquid in which KF ionizes. KF is a carrier of current)
At Anode
2F– → F2 + 2e–
At Cathode
2K + 2e– → 2K
2K + 2HF → 2KF + H2 ↑
As only HF is used up in the process, it is added periodically.
Drawbacks of Dennis Method:
- F2 liberated at the anode is usually contaminated with HF and CF4
- The graphite electrodes are continuously eaten up by fluorine and hence electrodes have to be periodically replaced.
- Molten electrolyte froths or foams. This foam or froth rises or creeps up the slanted V-shaped tube and chokes the outlets. This leads to an explosion due to the mixing of H2 and F2.
- Current efficiency in this method is low i.e. about 30%.
- At the high temperature (523 K) at which electrolysis is carried out, the attack of fluorine on the cell is vigorous.
Note:
Graphite is not suitable electrode at anode because it combines with fluorine formed and produces CF4. Hence it is necessary to replace graphite electrode time to time. The reaction is as follows
C + 2F2 → CF4
(Carbon) (Carbon tetrafluoride)
Whitlaw Gray’s Method:
Principle:
Pure, dry and anhydrous potassium hydrogen fluoride is electrolyzed in the molten state at 523 K. When hydrogen is liberated at the cathode while F2 is liberated at the anode.
Diagram:
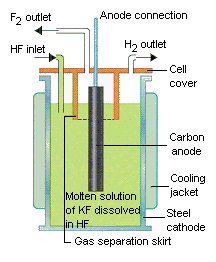
Whytlaw-Gray Cell (Apparatus):
The cell construction is modified to overcome some disadvantages of the ‘Dennis method”. Three main modifications are The cylindrical vessel, the vertical walls of this vessel minimize the creeping of the electrolyte. The diaphragm between electrodes prevents the mixing of Hydrogen and Fluorine. Blocking of exit tubes is avoided since they are with a large bore.
Electrodes: The cell consists of a rectangular copper vessel wound for electrical heating. This copper vessel act as a cathode. A pure thick graphite rod suspended at the centre serves as an anode. Anode and cathode are separated by a diaphragm. The anode is cemented to and insulated from the diaphragm cell by a paste of fluorspar CaF2. and water glass Na2SiO3. The diaphragm is closed at the bottom and it is laterally perforated below the level of the electrolyte. The upper solid non-porous part leads to a delivery tube (with large bore) for F2, gas.
Electrolyte: Pure, dry and anhydrous KHF2, in a molten condition. Anhydrous HF Is added periodically.
Temperature: 523 K
Heating unit: The copper vessel is covered from outside, with asbestos cement insulator of current but conductor of heat. A resistance wire for electrical heating and lagging – to prevent loss of heat by radiation.
Outlets: The outer copper vessel has an outlet for hydrogen and Inner copper diaphragm vessel has an outlet for fluorine gas. The outlets have large bore.
Current and Voltage: A current of 12 to 15 amperes and at 15 volts is employed.
Reactions:
2KHF2 → 2KF + 2HF
2KF → 2K+ + 2 F–
(HF acts as ionising liquid in which KF ionises. KF is a carrier of current)
At Anode
2F– → F2 + 2e–
At Cathode
2K + 2e– → 2K
2K + 2HF → 2KF + H2 ↑
As only HF s used up in the process, it is added periodically.
Purification:
F2 gas obtained by Dennis method contains two impurities (a) HF and (b) CF4. To purify fluorine, the gas is passed through copper U tubes containing dry NaF which absorbs HF vapours to form sodium hydrogen fluoride.
NaF(s) + HF(g) → NaHF2
Carbon tetrafluoride is condensed by cooling the gas in a trap cooled in liquid oxygen (90 K). Only CF4 gets condensed and is removed. Fluorine (BP 86 K) remains uncondensed and is collected in pure form.
Collection and Storage:
Fluorine gas is heavier than air. It is collected by upward displacement of air in copper cylinders. Fluorine is then compressed and stored In Cu-Ni alloy cylinders.
The Advantages of Whytlaw Gray’s Method over Denis Method:
- Fluorine liberated at the anode and hydrogen at the cathode are prevented from coming In contact with each other by the use of diaphragm cells.
- The walls of the electrolytic cell are vertical and broad outlets provided at the top of the containers for fluorine and hydrogen gas. The molten electrolyte cannot choke the outlets.
- The current efficiency is as high as 80%.
- This process is continuous.
Properties of Fluorine:
Physical properties of Fluorine:
- Colour: Fluorine is a pale yellow gas.
- Odour: It has an Irritating pungent odour.
- Nature: It Is highly poisonous and has a corrosive action on skin.
- Density: It Is heavier than air.
- Boiling point: 86 K
Chemical Reactions of Fluorine:
Fluorine is one of the most chemically active elements known since it reacts with almost all elements with the exception of nitrogen, oxygen, helium, and argon.
Reaction with Water:
Fluorine decomposes water at ordinary temperature forming hydrofluoric acid and liberating oxygen and ozone.
2H2O + 2F2 → 4HF + O2
3H2O + 3F2 → 6HF + O3
Reaction with cold dilute sodium hydroxide:
Cold dilute (2%) aqueous solution of NaOH gives sodium fluoride and oxygen difluoride.
2 NaOH + 2F2 → 2NaF + H20 + OF2
Sodium fluoride Oxygen difluoride
Reaction with hot concentrated sodium hydroxide:
Hot concentrated, aqueous solution of NaOH gives sodium fluoride and oxygen.
4 NaOH + 2F2 → 4NaF + 2H20 + 2O2
Reaction with Metals:
- With Sodium: Sodium burns spontaneously in fluorine to form sodium fluoride.
2Na + F2 → 2NaF
- With Magnesium: Magnesium reacts with fluorine on worming to form magnesium fluoride.
Mg + F2 → MgF2
- With Copper: Copper forms a thin layer of CuF2, on its surface which resists a further attack of fluorine.
Cu + F2 → CuF2
- With Mercury: Mercury reacts with fluorine to give mercury fluoride
Hg + F2 → HgF2
Reaction with Nonmetals:
- With Hydrogen: Fluorine reacts with Hydrogen with explosive violence even in dark and even at a low temperature as low as 63 K to form hydrogen fluoride.
H2 + F2 → 2HF
- With Boron :
2B + 3F2 → 2 BF3 (Boron trifluoride)
- With Carbon :
C + 2F2 → CF4 (Carbon tetrafluoride)
- With Silicon (Quartz):
Si + 2F2 → SiF4 (Silicon tetrafluoride)
- With Phosphorous :
2P + 3F2 → 2PF3 (Phosphorus trifluoride) and
2P + 5F2 → 2PF5 (Phosphorus pentafluoride)
- With Sulphur:
S + 3F2 → SF6 (Sulphur hexafluoride)
With chlorine Sulphur forms SCl4 and with fluorine, it forms SF6.
- Fluorine has the highest electronegativity and the smallest atomic size, due to which it is the strongest oxidizing agent. Hence it brings out the maximum oxidation state of any element.
- Hence with fluorine, every element shows a higher oxidation state. Hence with fluorine Sulphur shows its highest oxidation state of +6 while chlorine shows the +4 oxidation state.
Action of hydrocarbon (Methane):
Because of strong affinity of fluorine for hydrogen, fluorine decomposes hydrocarbons forming hydrogen fluoride and leaving behind carbon. Excess of fluorine react with carbon to form gaseous carbon tetrafluoride.
CH4 + 2F2 → 4 HF + C
Oxidising properties:
- Potassium Chlorate (KClO3):
KClO3 + F2 + H2O → 2HF + KClO4 (Potassium perchlorate)
- Potassium Sulphate (K2SO4)
K2SO4 + F2 → 2KF + K2S2O8 (Potassium persulphate)
- Potassium Carbonate (K2CO3) Potash ash:
K2CO3 + F2 → 2KF + K2C2O6 (Potassium percarbonate)
lnterhalogen Compounds of Fluorine:
Binary compounds of fluorine with other halogens are called interhalogen compounds of fluorine. In these compounds, fluorine shows -1 oxidation state. The other halogens show positive oxidation states such as +1, +3, +5, +7.
Fluorine can form four different types of binary compounds (fluorides) with other halogen and they are as follows.
| Type | XF | XF3 | XF5 | XF7 |
| With Cl | ClF | ClF3 | – | – |
| With Br | BrF | BrF3 | BrF5- | – |
| With I | – | – | IF5 | IF7 |
Action of Fluorine on Chlorine:
When chlorine and fluorine are made to react with each other in equal volumes, chlorine monofluoride is formed
Cl2 + F2 → 2CIF (Chlorine monofluoride)
When chlorine and excess fluorine are made to react, chlorine trifluoride is formed
Cl2 + 3 F2 → 2CIF3 (Chlorine trifluoride)
Action of Fluorine on Bromine:
When fluorine is made to react with bromine diluted with nitrogen, bromine monofluoride is formed
Br2 + 3F2 → 2BrF3 (Bromine trifluoride)
When excess fluorine is made to react with bromine, bromine pentafluoride is formed.
Br2 + 5 F2 → 2BrF5 (Bromine pentafluoride)
Action of Fluorine on Iodine:
When excess fluorine is made to react with iodine, iodine pentafluoride is formed
I2 + 5F2 → 2IF5 (Iodine pentafluoride)
When much excess fluorine is made to react with iodine, iodine heptafluoride is formed
I2 + 7 F2 → IF7 (Iodine heptafluoride)
Uses of Fluorine:
- Freon: It is used in the preparation of dichloro-difluoro-methane, CCl2F2, known as ‘Freon’. Freon is used in refrigeration and air-conditioning.
- Teflon: It is used to prepare Teflon. Teflon is polymerized tetrafluoroethylene. Teflon is a chemically inert and electrical insulator. It is used for coating frying pans, cooking pots, and reaction vessels.
- UF6: It is used for the preparation of UF6. UF6 is helpful in the separation of isotopes of Uranium.
- SF6: It is used for the preparation of SF6. SF6 is used in the vulcanization of rubber and for high voltage insulation.
- Rocket Fuel: In combination with hydrazine (N2H4), fluorine is used as rocket fuel.
Prevention of Tooth Decay or Dental Decay:
The tooth enamel is composed of Ca5(OH)(PO4)3 which slowly converts to Ca5F(PO4)3 due to the regular brushing of teeth with the fluoride toothpaste. (The fluoride ion is added in the form of soluble SnF2. or sodium mono-fluoro-phosphate to toothpaste).
The fluoro compound Ca5F(PO4)3 resists the attack of germs and mild organic acids associated with foodstuff and prevents tooth decay.
One reply on “Fluorine”
its really interesting