Science > Chemistry > Biomolecules > Disaccharides and Polysaccharides
In the last article, we have studied monosaccharides. In this article, we shall study disaccharides and polysaccharides.
Disaccharides:
Di-saccharides on hydrolysis give two molecules of monosaccharide. They on hydrolysis with dilute acids or enzymes yield two molecules of either the same or different monosaccharides.
e.g. Cane sugar (Sucrose) (C12H22O11)on hydrolysis gives one molecule of glucose and one molecule of fructose, Maltose (C12H22O11) on hydrolysis gives two molecules of glucose, Lactose (C12H22O11) on hydrolysis gives one molecule of glucose and one molecule of galactose.
- Disaccharides are crystalline, water-soluble, and sweet in taste.
- They have the general formula (C12H22O11).
- The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
Examples of Disaccharides:
Sucrose:
One of the common disaccharides is sucrose which on hydrolysis gives an equimolar mixture of α -D-Glucapyranose and β-D-Fructofuranose. These two monosaccharides are held together by a glycosidic linkage between C1 of α-glucose and C2 of β-fructose.
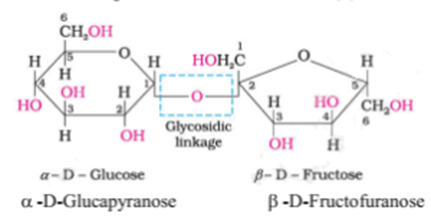
Since the reducing groups of glucose and fructose are involved in the glycosidic bond formation, sucrose is a non-reducing sugar.
Sucrose is dextrorotatory but after hydrolysis gives dextrorotatory glucose and laevorotatory fructose. Since the laevorotation of fructose (–92.4°) is more than dextrorotation of glucose (+ 52.5°), the mixture is laevorotatory. Thus, hydrolysis of sucrose brings about a change in the sign of rotation, from dextro (+) to laevo in the sign of rotation, from Dextro (+) to Laevo (–) and the product is named as invert sugar.
Maltose:
Another disaccharides, maltose is composed of two α-D-glucose units in which C1 of one glucose (I) is linked to C4 of another glucose unit (II).
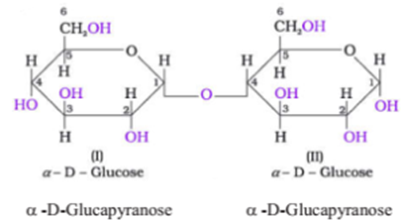
The free aldehyde group can be produced at C1 of second glucose in solution and it shows reducing properties so it is a reducing sugar
Cellobiose:
Cellobiose is obtained by partial hydrolysis of cellulose, C1 of one β-D-Glucapyranose is linked to C4 of another β-D-Glucapyranose by glucosidic linkage. Thus cellobiose contains 1→ 4 β- glucosic bond.
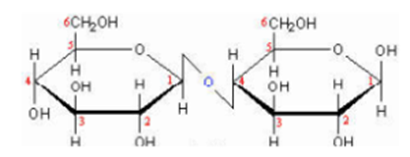
Cellobiose is reducing sugar because free aldehyde group can be produced at C1 in second glucose molecule
Lactose:
It is more commonly known as milk sugar since this disaccharide is found in milk. It is composed of β-D-galactose (β-D-Galactopyranose) and β-D-glucose (β-D-Glucopyranose). The glucosidic linkage is between C1 of β-D-galactose (b-D-Galactopyranose) and C4 of β-D-glucose (β-D-Glucopyranose). Hence it is also a reducing sugar.

Reducing Sugars:
The saccharides in which aldehydic and ketonic groups are free are called reducing sugars. They reduce Fehling’s solution and Tollen’s reagent. They contain either α-hydroxy aldehyde or α -hydroxy ketone group or contain cyclic hemiacetal or cyclic hemiketal structures.
All monosaccharides are reducing sugars. Diasaccharides like maltose, lactose and cellobiose are reducing sugars.
Nonreducing sugars:
The saccharides in which aldehydic and ketonic groups are not free are called non-reducing sugars. They do not reduce Fehling’s solution and Tollen’s reagent. They contain stable acetal or ketal structures which cannot be opened into the free carbonyl group.
Sucrose, Starch, Cellulose, Glycogen, Dextrin are reducing sugars.
Hemiacetal and Hemiketal Structures:
They are formed when an alcohol oxygen atom adds to the carbonyl carbon of an aldehyde or a ketone. When alcohol adds to an aldehyde, the result is called a hemiacetal; when alcohol adds to a ketone the resulting product is a hemiketal.
This happens through the nucleophilic attack of the hydroxyl group at the electrophilic carbonyl group. Since alcohols are weak nucleophiles, the attack on the carbonyl carbon is usually promoted by protonation of the carbonyl oxygen.
PolySaccharides:
Carbohydrates which on hydrolysis give indefinite or large no. of monosaccharides (more than 10) are called polysaccharides. They contain a large number of monosaccharide units joined together by glycosidic linkages. These are the most commonly encountered carbohydrates in nature. They mainly act as food storage or structural materials.
- They are natural polymeric carbohydrates. They are insoluble in water, amorphous, and tasteless.
- They are nonsugars.
- They have the general formula (C6H10O5)n. e.g. Starch, Cellulose, Inulin, Dextrin, etc
- Cellulose is a linear polymer of β-Glucose units while starch is a branched polymer of a-glucose units.
Examples of Polysaccharides:
Starch:
Starch is the main storage polysaccharide of plants. It is the most important dietary source for human beings. High content of starch is found in cereals, roots, tubers, and some vegetables. It is a polymer of α-glucose (α-D-Glucopyranose) and consists of two components Amylose and Amylopectin.
Amylose is a water-soluble component which constitutes about 15-20% of starch. Chemically amylose is a long unbranched chain with 200-1000 α-D-(+)-glucose units held by C1– C4 glycosidic linkage.
Amylopectin is insoluble in water and constitutes about 80-85% of starch. It is a branched chain polymer of α-D-glucose units in which chain is formed by C1–C4 glycosidic linkage whereas branching occurs by C1–C6 glycosidic linkage


Glycogen:
The carbohydrates are stored in the animal body as glycogen. It is also known as animal starch because its structure is similar to amylopectin and is rather more highly branched. It is present in the liver, muscles, and brain. When the body needs glucose, enzymes break the glycogen down to glucose (hydrolysis). Glycogen is also found in yeast and fungi.
Importance of Carbohydrates:
- Carbohydrates are essential for life in both plants and animals.
- They form a major portion of our food. Honey has been used for a long time as an instant source of energy by ‘Vaids’ in Ayurvedic system of medicine.
- Carbohydrates are used as storage molecules as starch in plants and glycogen in animals.
- The cell wall of bacteria and plants is made up of cellulose. We build furniture, etc. from cellulose in the form of wood and clothe ourselves with cellulose in the form of cotton fibre.
- They provide raw materials for many important industries like textiles, paper, lacquers and breweries.
- Two aldopentoses viz. D-ribose and 2-deoxy D-ribose (Section are present in nucleic acids. Carbohydrates are found in biosystem in the combination with many proteins and lipids.