Science > Chemistry > Biomolecules > Glucose and Fructose
In the last article, we have seen what are carbohydrates and how are they classified. Based on hydrolysis behaviour the carbohydrates are classified into three types. a) Mono-Saccharides b) Oligo-Saccharides and c) Poly-Saccharides. In this article, we shall study monosaccharides in detail particularly glucose and fructose.
Monosaccharides:
Carbohydrates which are basic units or which cannot be hydrolyzed further are called monosaccharides.
Characteristics of mono-saccharides:
- Carbohydrates which are basic units or which cannot be hydrolysed further are called monosaccharides.
- They are basic units of carbohydrates.
- They cannot be hydrolysed further into small units.
- They contain six carbon atoms in a molecule.
- They are water-soluble and sweat in taste.
- Depending upon the presence of an aldehydic group or a ketonic group they are further subclassified into aldoses and ketoses respectively.
Aldoses: The monosaccharides containing the aldehydic group are called aldoses. Examples: Aldopentose C5H10O5 – Arbaniose, Xylose, Ribose. Aldohexose C6H12O6 – Glucose, Galactose
Ketoses: The monosaccharides containing the ketonic group are called ketoses. Examples: Ketopentose C5H10O5 – Ribulose.Ketohexose C6H12O6 – Fructose.
Preparation of Glucose:
From Sucrose (Cane sugar): (Laboratory Method):
When powdered cane sugar is heated with a concentrated alcoholic solution of HCl on a water bath for about 2 hours at about 323 K, glucose is formed.
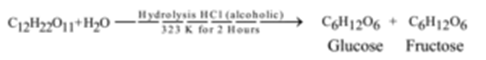
It is insoluble in alcohol while fructose is soluble in alcohol. Hence glucose crystallizes out first leaving fructose in the solution. A few crystals of glucose may be added to the solution for quicker crystallization. This is known as seeding. Purification is done by recrystallizing it from methanol.
From Starch: (Commercial Method):
Starch on hydrolysis with dilute sulphuric acid by heating under 3 to 5 atmospheric pressure gives glucose.
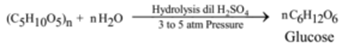
When hydrolysis is complete the excess of unreacted sulphuric acid is neutralised with calcium carbonate and filtered to remove the precipitate of calcium sulphate.
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
The filtrate which contains glucose and one molecule of water is decolorized using animal charcoal. The clear solution is then evaporated in a vacuum to get a thick syrup on cooling crystallizes to give glucose-monohydrate. It is recrystallized from methanol.
Structure of Glucose:
Open Chain Structure:
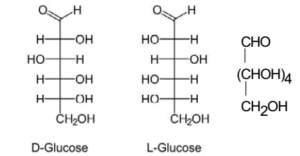
Following chemical reactions of glucose confirm its open chain structure
- On prolonged heating with HI it gives n-hexane suggesting that all the six carbons are linked in a straight chain.

- Hydroxylamine condenses with an aldehydic group to form glucose-oxime.

- Hydrogen cyanide adds to an aldehydic group to form cyanohydrin.

- On oxidation by a mild oxidizing agent like bromine water, it gives gluconic acid, which shows that the carbonyl group is the aldehyde group.

- Glucose, as well as gluconic acid on oxidation by dilute nitric acid, gives dicarboxylic acid, saccharic acid, which shows the presence of the alcoholic group.

- On acetylation by acetic anhydride it gives glucose-pentaacetate., which confirms the presence of five hydroxyl group. As glucose is a stable compound these five hydroxyl group must be on five different carbon atoms.

Challenges to open chain structure or the need of cyclic structure:
The following points indicate the absence of a free aldehyde group in glucose.
- In spite of having an aldehyde group it does not give a condensation reaction with 2,4 dinitro-phenyl hydrazine
- Glucose-pentaacetate does not condense with hydroxylamine
- It is found to exist in two different crystalline forms α and β called anomers.
Haworth Projection of Glucose:
The cyclic structure in Haworth projection depicts the ring as being flat. The substituents that are to the right in a Fischer projection formula are down and those to the left are up in the corresponding Haworth projection formula. Orient the Haworth projection formula with the ring oxygen at the back and the anomeric carbon at the right.
For carbohydrates of D series a) If hydroxyl is down, the configuration of anomeric carbon is α and b) If hydroxyl is up, the configuration of anomeric carbon is β.
For carbohydrates of L series a) If hydroxyl is up, the configuration of anomeric carbon is α and b) If hydroxyl is down, the configuration of anomeric carbon is β.

Note: A ring containing five carbons and one oxygen is referred pyran
Physical Properties of Glucose:
- It is a white crystalline solid.
- It is soluble in water but sparingly soluble in alcohol.
- As it contains 4 asymmetric carbon atoms, it is an optically active compound.
- It is dextrorotatory. It has a specific rotation of + 52.5°.
Fructose::
Fructose also has the molecular formula C6H12O6 and on the basis of its reactions, it was found to contain a ketonic functional group at carbon number 2 and six carbons in the straight chain as in the case of glucose.
It belongs to D-series and is a laevorotatory compound. It is appropriately written as D-(–)-fructose. Its open chain structure is as shown.

One reply on “Glucose and Fructose”
Well explained… Helped me on my assignment. Thank you