Science > Chemistry > Biomolecules > Introduction to Carbohydrates
All those simple or complex organic and inorganic substances found in different living forms are collectively called as biomolecules. Biomolecules can be grouped into two types micro and macromolecules. The micro molecules have moderate to low molecular mass. e.g. sugars, amino acids, and their derivatives, vitamins, coenzymes, nucleotides, inorganic salts, and water. The macromolecules have very large molecular mass. e.g. carbohydrates, proteins, fats, oils, lipids, and nucleic acids. These biomolecules contribute in one or other way to the structure and/or function of the living system.
Biomolecules are the lifeless molecules which combine in a specific manner to produce life or control biological reactions. e.g. carbohydrates, proteins, fats, oils, lipids, and nucleic acids.
Carbohydrates are polyhydroxy aldehydes or polyhydroxy ketones or compounds that give polyhydroxy aldehydes or polyhydroxy ketones on hydrolysis. They contain at least one asymmetric carbon atom. e.g. Glucose (C6H12O6), Cane sugar Sucrose (C12H22O11). They are a class of naturally occurring organic compounds found in plants and animal kingdoms. They contain carbon, hydrogen, and oxygen actually carbohydrates mean hydrates of carbon. The proportion of hydrogen and oxygen is definite 2:1. The exception is Rhamnose (C6H12O5). The sources of carbohydrates are Rice, Wheat, Jowar, Bajra, etc. They function as structural material (Cellulose) and reserved food material (Sugar, starch). They are essential for the growth and maintenance of animal life.
Classification of Carbohydrates:
On the Basis of Solubility and Taste:
Based on solubility and the taste, the carbohydrates are classified into two types. a) Sugars and b) Non-sugars.
Characteristics of sugars:
- They are soluble in water.
- They are crystalline in nature.
- They are sweet in taste.
- e.g. Cane sugar, Glucose etc.
Characteristics of non sugars:
- They are insoluble in water.
- They are amorphous in nature.
- They are tasteless
- e.g. Starch, cellulose, etc.
On the Basis of Hydrolysis Behaviour:
Based on hydrolysis behaviour the carbohydrates are classified into three types. a) Mono-Saccharides b) Oligo-Saccharides and c) Poly-Saccharides.
Monosaccharides:
Carbohydrates which are basic units or which cannot be hydrolyzed further are called monosaccharides.
Characteristics of mono-saccharides:
- Carbohydrates which are basic units or which cannot be hydrolysed further are called monosaccharides.
- They are basic units of carbohydrates.
- They cannot be hydrolysed further into small units.
- They contain six carbon atoms in a molecule.
- They are water-soluble and sweat in taste.
- Depending upon the presence of an aldehydic group or a ketonic group they are further subclassified into aldoses and ketoses respectively.
Aldoses: The monosaccharides containing the aldehydic group are called aldoses. Examples: Aldopentose C5H10O5 – Arbaniose, Xylose, Ribose. Aldohexose C6H12O6 – Glucose, Galactose
Ketoses: The monosaccharides containing the ketonic group are called ketoses. Examples: Ketopentose C5H10O5 – Ribulose.Ketohexose C6H12O6 – Fructose.
Oligo-saccharides:
Carbohydrates which on hydrolysis give definite no. of monosaccharides i.e. 2 to 9 molecules of monosaccharides are called oligosaccharides. Depending upon no. of monosaccharides formed on hydrolysis they are further subclassified into Di-saccharides, Tri-saccharides, Tetra-saccharides, etc.
Di-saccharides:
- Di-saccharides on hydrolysis give two molecules of monosaccharide.
- e.g. Cane sugar (Sucrose) (C12H22O11)on hydrolysis gives one molecule of glucose and one molecule of fructose, Maltose (C12H22O11) on hydrolysis gives two molecules of glucose, Lactose (C12H22O11) on hydrolysis gives one molecule of glucose and one molecule of galactose.
- Disaccharides are crystalline, water-soluble and sweet in taste.
- They have the general formula (C12H22O11).
Tri-saccharides:
- Tri-saccharides on hydrolysis give three molecules of monosaccharide.
- e.g. Raffinose (C18H32O16).
Tetra-saccharides:
- Tetra-saccharides on hydrolysis give three molecules of monosaccharide.
- e.g. Stachyose (C24H42O21).
Poly-saccharides:
- Carbohydrates which on hydrolysis give indefinite or large no. of monosaccharides (more than 10) are called polysaccharides.
- They are natural polymeric carbohydrates. They are insoluble in water, amorphous and tasteless.
- They are nonsugars.
- They have the general formula (C6H10O5)n. e.g. Starch, Cellulose, Inulin, Dextrin etc
- Cellulose is a linear polymer of β-Glucose units while starch is a branched polymer of a-glucose units.

Action of Fehling’s solution on Sucrose:
- Sucrose gives polyhydroxy aldehydes on hydrolysis.
- Fehling’s solution is a mild oxidising agent. It is a complex of cuprous ions of tartaric acid in the presence of an alkali.
- When treated with hydrolysis products of sucrose, the aldehyde formed gets oxidised and the red precipitate of cuprous oxide is formed.
Fischer Projection:
The wedge and dash representations of stereochemistry can often become cumbersome, especially for large molecules which contain a number of stereocenters. An alternative way to represent stereochemistry is the Fischer Projection, which was first used by the German chemist Emil Fischer. The Fischer projection represents every stereocenter as a cross. The horizontal line represents bonds extending out of the plane of the page, whereas the vertical line represents bonds extending into the plane of the page.
When working with Fischer Projections, keep in mind the following rules:
- Because the “up” and “down” aspects of the bonds don’t change, a Fischer projection may be rotated by 180 degrees without changing its meaning.
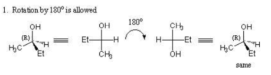
- A Fischer projection may not be rotated by 90 degrees. Such a rotation typically changes the configuration to the enantiomer.
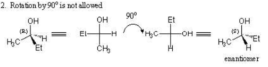
- To find the enantiomer of a molecule drawn as a Fischer projection, simply exchange the right and left horizontal bonds.

- To determine whether the molecule in Fischer projection is a meso compound, draw a horizontal line through the center of the molecule and determine whether the molecule is symmetric about that line.

- D – and L – Sugars: Glyceraldehyde is an aldotriose it is the simplest optically active compound. It contains one asymmetric carbon atom and has two enantiomers (stereoisomers) as shown

- In Fischer projection, if the hydroxyl group at the lowest chirality centre points to the right, the monosaccharide is referred as D- sugar. if the hydroxyl group at the lowest chirality centre points to the left, the monosaccharide is referred as D- sugar.

Structure of Some Monosaccharides:
Triose (C3H6O3):

Tetrose (C4H8O4):

Pentose (C5H10O5):

Hexose (C6H12O6):
