Isotopes are different atoms of the same elements having the same atomic number but a different mass number. Isotopes of radioactive elements are found to be very useful in different fields like carbon dating, agriculture, medicines, the production of synthetic elements, etc. Important uses of radioisotopes are as follows.
Estimation of Age of Rock and Bone Samples:
Estimation of Earth’s Age (Uranium Dating Technique):
The radioactive uranium (238U )ore is found with nonradioactive lead (206Pb). The end product of radioactive decay of uranium is lead. A sample of radioactive ore of uranium is obtained and is analyzed for the radioactive uranium and nonradioactive lead.
Let the quantities, N = 238U moles, No = 238U moles+ 206Pb moles
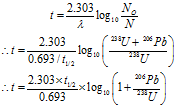
Knowing the mole ratio (206Pb/238U) in the sample of the ore. The age of the earth can be estimated. It is about 4.5 billion years.
Example 01:
The mass ratio (206Pb/238U) in the sample of the ore is 0.008:1. Estimate the age of the mineral. Take the half-life period of uranium as 4.51 billion years.
Solution:
Given: The mass ratio 206Pb : 238U = 0.008:1, Half life period = t1/2 = 4.51 billion years = 4.51 × 109 years
To Find: Age of mineral = t = ?
The mass ratio is 206Pb : 238U = 0.008:1
Their mole ratio is (0.008 / 206) : (1/238) = 0.0092 : 1
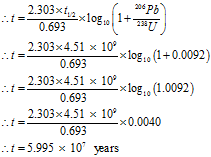
Example 02:
On analysis of a rock sample, the relative numbers of atoms of 206Pb and 238U is 0.25. Estimate the age of the rock. Take the half-life period of uranium as 4.51 billion years.
Solution:
Given: The atom ratio 206Pb : 238U = 0.25, As they are monoatomic elements, their mole ratio is same as their atomic ratio. Half-life period = t1/2 = 4.51 billion years = 4.51 × 109 years
To Find: Age of rock = t = ?
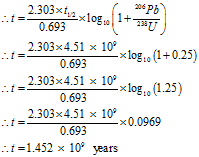
Example 03:
On analysis of a rock sample, the molar proportion of uranium to lead is 1:3. Estimate the age of the rock when it was lead-free. Take the half-life period of uranium as 4.5 billion years.
Solution:
Given: The mole ratio 206Pb : 238U = 3:1, Half-life period = t1/2 = 4.51 billion years = 4.51 × 109 years
To Find: Age of rock = t = ?

The age of the rock is 1.50 x 1010 years
Carbon Dating:
Radioisotope 14C is used to estimate the age of archaeological and biological specimens. This technique was first time developed by W. F. Libby. The process in which age of fossils of plant or animal origin is determined by measuring the quantity of 14C present in the sample is called carbon dating.
The living matter always contains a definite amount of radioactive carbon 6C14. Due to the interaction between neutrons produced by cosmic rays and atmospheric 14N, radioactive 14C is formed.

Half-life period of 14C is redefined by IUPAC at 5730 ± 40 years. This radioactive carbon reacts with atmospheric oxygen and radioactive carbon dioxide is produced. This radioactive CO2 enters in living plants with normal CO2 which is used by plants to prepare carbohydrates by photosynthesis. These carbohydrates are then consumed by living animals. During the life cycle of the living organism, 14C decays into 12Cbut it is again made up. A state of equilibrium is ultimately attained and a living plant or an animal on an average gives 15.3 disintegrations per minute per gram. Once the animals or plants are dead, 14C isotope will start disintegrating in them without the intake. Hence 14C count of dead plants or animals will be the measure of time lapsed since the animals or plants were alive. These counts are compared with that in the environment.
In the carbon dating method sample of dead tissue is burnt to obtain carbon dioxide and the ratio of 14C to 12C is obtained and probable age can be calculated using the formula.


The rapid disintegration of 14C generally limits the dating period to approximately 50000 years. Thus carbon dating method cannot be used to find the age of samples older than 50,000 years.
Example 04:
In the carbon dating method, the amount of 14C isotope in a piece of wood is found to be one-fifth of that present in a fresh piece of wood. If the half-life period of 14C is 5577 years, calculate the age of the wood piece.
Solution:
Given: N = 1/5 N0, Half-life period = t1/2 = 5577 years
To Find: Age of wood piece = t = ?

Example 05:
A wooden sample gave an activity of 16 β particles per second while freshly cut wood samples gave an activity of 64 β particles per second. If the half-life period of 14 C is 5770 years, find the age of the wooden sample.
Solution:
Given: N = 16 β particles per second, N0 = 64 β particles per second, Half-life period = t1/2 = 5770 years
To Find: Age of wood piece = t = ?

Short Cut: to reduce activity from 64 β particles per second to 32 β particles per second it will take 5770 years and to reduce activity from 32 β particles per second to 16 β particles per second it will take another 5770 years. Thus totally it will take 11540 years.
Potassium-Argon Dating:
This method is used by geologists for dating very old archaeological materials and rocks. They have used this method to date rocks as much as 4 billion years old. The principle of the method is that some of the radioactive isotopes of Potassium, Potassium-40 (40K), decays to the gas Argon as Argon-40 (40Ar). Potassium (K) is one of the most abundant elements in the Earth’s crust (2.4% by mass) obtained in mica, feldspar and hornblendes. One out of every 10,000 Potassium atoms is radioactive Potassium-40 (40K). They each have 19 protons and 21 neutrons in their nucleus. If one of these protons is hit by a beta particle, it can be converted into a neutron. With 18 protons and 22 neutrons, the atom has become Argon-40 (Ar-40), an inert gas. For every 100 K-40 atoms that decay, 11 become Ar-40.
By comparing the proportion of 40K to 40Ar in a sample of the volcanic rock, and knowing the half-life of 40K, the date that the rock formed can be determined.
The temperature inside the earth is very high. The leakage of argon through the rock takes place at 125 °C. It creates a problem to find the actual ratio of 40K to 40Ar in the sample.
Rubidium-Strontium Dating:
This method is used by geologists for dating estimating the age of rocks, minerals, and meteorites from measurements of the amount of the stable isotope strontium-87 formed by the decay of β particle of the unstable isotope rubidium – 87. The method is applicable to very old rocks because the transformation is extremely slow: the half-life of rubidium-87 to is approximately 50 billion years.
The advantage of this method over potassium-argon method is that strontium formed does not diffuse through rocks as organ does.
Most minerals that contain rubidium also have some strontium incorporated when the mineral was formed, so a correction must be made for this initial amount of strontium to obtain the radiogenic increment.
In Production of Synthetic Elements:
The elements after uranium in the periodic table are called trans-uranic elements. They are prepared by nuclear transmutation in laboratories.
- When uranium 92U238 isotope is bombarded by a high-speed neutron, neptunium is obtained.

Neptunium emits β particle giving rise to plutonium.

- When uranium 92U238 isotope is bombarded by high-speed alpha particles, Plutonium is obtained.

Plutonium emits β particle giving rise to Armecium.

In Medicine:
- Many radioisotopes are now widely used by the medical field to detect and to cure them.
- In cancer treatment, C60 is used to destroy malignant cells. C60 gives β particles and strong ϒ rays which are used to destroy malignant cells. This treatment is called radiotherapy.
- Radioactive isotope I131 is used for the detection of a brain tumour.
- Radioactive isotope I131 is used in the detection and cure of thyroid gland diseases.
- Radioactive isotope P32 is used in the treatment of leukemia (blood cancer).
- Radioactive isotopes are used to study metabolic processes in human bodies. When used for this purpose they are called tracers.
- Radioactive isotopes are used to study the mechanism of action of drugs.
- Na24 isotope is used to examine blood circulation. An appropriate quantity of NaCI solution containing Na24 is injected into the patient’s arm. A Geiger counter is placed in contact with one of the feet. Na24 reaches the feet in about 30 minutes, if the circulation of blood is normal, while restricted circulation takes more time. In this way, the obstruction can be located by moving the Geiger counter over different parts of the body.
In Agriculture:
- Radioisotopes are used to study plant metabolism. e.g. Ca20 and P32 are used to study plant metabolism.
- Co60 is used for irradiation which is beneficial in the production of carrot seeds.
- S35 isotope is used in the study of the effect of many fungicides on plants.
- Radiations from radioactive isotopes accelerate the growth of plants and yield of the crops.
- Sprouting of potatoes and onions is checked (avoided) by irradiation by radiations given out by a radioactive isotope.
- Proper irradiation of food and food grain increase their storage life.
- The radioactive isotope of carbon is used to study photosynthesis in plants.
- Radiations from radioisotopes are used to destroy pests and insects on plants.