Science > Chemistry > Introduction to Organic Chemistry > Chemistry of Carbon Compounds
Organic chemistry is a branch of chemistry, which studies carbon compounds. Organic substances are substances derived from organisms like plants and animals. In the olden days it was assumed that these substances cannot be prepared in laboratories. Berzelius assumed that some vital force was necessary for the production of these organic compounds. He assumed that living beings possess this vital force (life force). Hence it is not possible to produce organic substances in laboratories. Now the word organic has no significance as these substances can be prepared in laboratories.
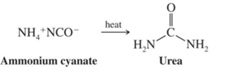
In 1828 Wohler synthesized first organic compound, urea in the laboratory by heating an inorganic compound ammonium cyanate. It is molecular rearrangement process.
In 1845 Kolbe synthesized acetic acid, from its elements. In 1856 Berthelot synthesized methane in the laboratory.
Carbon compounds are compounds in which carbon is combined with elements such as hydrogen, oxygen, nitrogen, halogens, sulphur, phosphorous and few metals. They may be derived from living organisms like plants and animals or they may be prepared in laboratories (Synthetic substances).
The simplest organic compounds are hydrocarbons. Other organic compounds can be derived from hydrocarbons. Hence in simple words, we can define organic chemistry as the study of hydrocarbons and their derivatives.
The Necessity of Separate Branch for Study of Carbon Compounds:
Organic chemistry is a branch of chemistry, which studies carbon compounds. The necessity of separate branch of carbon compounds can be explained as follows.
- Organic chemistry is the largest part of chemistry.
- The number of carbon compounds far exceeds the number of compounds of all the remaining hundred and odd elements put together. Organic compounds comprise 90% of all known compounds. It is due to the special capability of carbon called catenation and the formation of isomers of the compounds.
- Carbon compounds form the basis of modern chemical industries.
- The food materials contain nutrients like fats, carbohydrates, proteins are carbon compounds.
- The fuels like petrol, diesel, compressed natural gas (C.N.G.), liquefied petroleum gas (L.P.G.) are carbon compounds.
- Fibres like wool, silk, cotton, jute, and synthetic fibres like nylon, terylene, polyester are carbon compounds.
- Paints, varnishes, dyes, perfumes, insecticides, fertilizers, soil conditioners, plastics, detergents, drugs are carbon compounds.
Thus there is no aspect of our material life that is not touched by organic chemistry
Carbon forms a Large Number of Compounds:
The number of carbon compounds far exceeds the number of compounds of all the remaining hundred and odd elements put together. It can be explained as follows.
- The atomic number of carbon is 6. The electronic configuration of carbon is 2,4. Thus each atom of carbon contains 4 electrons in the outermost orbit.
- The carbon atom can share its electrons with the atoms of different elements like H, O, N, S, P, Cl, Br, I, etc.
- Carbon possesses a property called catenation by which it has the capacity to form a direct bond with another atom of carbon to form chains or rings of different sizes and shapes. Two carbon atoms can form a single bond, double bond, or triple bond among themselves.
- Carbon has the capacity to get attached directly to active metals like magnesium to form organic metallic compounds.
- Compounds of carbon are obtained in different isomeric forms.
Catenation:
Catenation is the capacity to form a direct bond with another atom of the same element to form chains or rings of different sizes and shapes. Example bond formation between two carbon atoms. Besides carbon following elements show the capacity of catenation.
| Bond | C-C | Si-Si | S-S | P-P | N-N | O-O |
| Bond energy in Kcal mol-1 | 347.3 | 225.9 | 225.9 | 209.2 | 163.2 | 146.4 |
The bond energy is the highest in carbon and it decreases in order C > Si > S > P > N > O. The stability of the bond decreases in the same order. Hence the ability to form long chains decreases in the same order.
Sources of Organic Compounds:
Natural Sources:
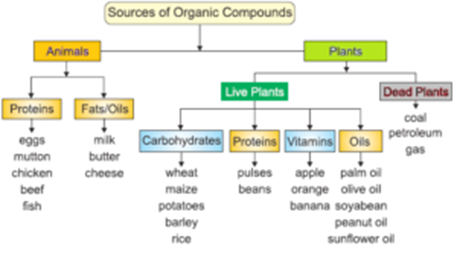
- Fungi and microorganisms produce alcohols, acids, vitamins and antibiotics during fermentation.
- From coal by a destructive distillation aromatic hydrocarbons, dyes, drugs, perfumes are obtained.
- Natural gas and petroleum is a major source of organic compounds like gasoline, kerosene, lubricants, machine oils, paraffin waxes, vaseline, etc.
- 10 % of organic compounds are sourced from natural sources.
Synthetic Sources:
Man-made or artificially prepared substances are called synthetic substances. Simple organic substances are obtained from petroleum and then applying certain processes they are converted into complex organic substances in industry or laboratories. 90 % of organic compounds are sourced from synthetic sources.
The Significance of Organic Compounds in Modern Life:
The significance of organic compounds in modern life can be explained w.r.t. following industries.
- Food Industry: The food materials contain nutrients like fats, carbohydrates, proteins are organic compounds. Artificial sweetener, flavouring agent, food preservatives are organic compounds.
- Petroleum Industry: The fuels like kerosene, gasoline, petrol, diesel, compressed natural gas (C.N.G.), liquefied petroleum gas (L.P.G.) are organic compounds. Paraffins (Wax), Vaseline, Boot Polish etc. are organic compounds.
- Textile Industry: Fibres like wool, silk, cotton, jute are organic compounds. Synthetic fibres like nylon, terylene, polyester are organic compounds.
- Solvent Industry: Water is a good solvent but there are many substances which are not getting dissolved in water. Organic substances like chloroform, alcohol, benzene, acetone, carbon tetrachloride are good solvents.
- Plastic Industry: Polyvinyl chloride (PVC), polyethene, bakelite, rubber are organic compounds.
- Paints and Dyestuff Industry: Paints, varnishes, dyes, indigo, azo dyes, printing inks are organic compounds. e.g. Malachite gree, Alizarin, etc.
- Soap and Detergent industry: Detergents are organic compounds. soaps are alkali salts of higher fatty acids are organic compounds.
- Miscellaneous Industry: Perfumes, insecticides (D.D.T., Gammexane, Malathion, etc.), fertilisers like urea, drugs are carbon compounds.
- Medicines: Antibiotics Penicillin, Streptomycin, Chloromycetin, Sulphadiazine, Aspirin, Morphine, Cocaine, iodoform are organic compounds,
- Explosives: Nitroglycerine, Nitrocellulose, T.N.B., T.N.T., etc. are organic compounds.
Characteristics of Organic Compounds:
- Types of bond and linkage: Organic compounds are generally covalent compounds and do not ionize when dissolved in water.
- Solubility: Organic compounds are mostly insoluble in water. They are soluble in organic solvents like benzene, alcohol, chloroform.
- Melting and Boiling points: Organic compounds have generally low melting points and boiling points.
- Electrical Conductivity: Organic compounds are bad conductors of electricity.
- Isomerism: Two or more compounds having the same molecular formula but different structural formulae are called isomers and the phenomenon is known as isomerism. Isomerism is a unique characteristic of organic compounds.


- Polymerization: Organic compounds have a tendency to form a polymer.
- Nature: Organic compounds have a well-established structure and are complex compounds and possess high molecular weight.
- Odour: Many organic substances have a characteristic odour. e.g. esters have a sweet odour, amines have a fishy odour, phenol has a clinical smell.
- Rate of Reaction: Reactions involving organic compounds are slow as breaking and forming of covalent bonds takes place.