Science > Chemistry > Organic Chemistry > Halogen Derivatives of Alkanes > Preparation of Alkyl halides From Alcohols
In the last two articles, we have studied the methods of preparation of alkyl halides from alkanes and alkenes. In this article, we shall study the preparation of alkyl halides from alcohols.
The Action of HX on Alcohols:
- The reaction is nucleophilic substitution.
- Methyl and primary alcohol undergo SN¹ mechanism while secondary and tertiary alcohols undergo SN² mechanism.
- Among halogen halides, HCl is the least reactive in nature. Because the chloride ion is a weaker nucleophile than bromide or iodide ions. Hence hydrogen chloride is mixed with anhydrous ZnCl2.
- In these reactions, anhydrous ZnCl2 not only acts as a dehydrating agent but also helps in the cleavage of C-O bond of the alcohol. ZnCl2 is Lewis acid and coordinates with the oxygen atom and thus weakens C-O bond. This results in the formation of carbocation which combines with Cl- ion to form chloroalkane. Tertiary alcohols are highly reactive hence for tertiary alcohol ZnCl2 is not required.
- This reaction is nucleophilic substitution. The stability of carbocations is of order tertiary > secondary > primary. Hence the order of reactivity also follows the same order tertiary > secondary > primary.
- The bond dissociation energy of H-X bond is of order H-Cl > H-Br > H-I. hence the reactivity of halogen acids follows the order HI > HBr > HCl.
- Unlike alkyl chlorides, the secondary and tertiary bromides and iodides cannot be obtained from their respective alcohols. It is because the secondary and tertiary alcohols on heating with concentrated H2SO4 undergo dehydration to form alkenes. Hence for these preparations dilute H2SO4 is used.
General Reaction:
R–OH + HX → R–X + H2O
Alcohol Halogen acid alkyl halide
Order of Reactivity:
Tertiary alcohol > Secondary alcohol > Primary alcohol
and HI > HBr > HCl.
By Action of Haloacids:
Preparation of Alkyl Chlorides:
General Reaction:
When alcohol is treated with Lucas reagent alkyl chloride is obtained. Lucas reagent is a solution of a concentrated hydrochloric acid with zinc chloride. This reaction is known as Groove’s process.
R–OH + HCl ![]() R–Cl + H2O
R–Cl + H2O
Alcohol Conc.Hydrochloric acid Alkyl chloride
Example – 1: Preparation of ethyl chloride (Chloroethane) from ethyl alcohol (Ethanol):
C2H5OH + HCl ![]() C2H5Cl + H2O
C2H5Cl + H2O
Ethyl alcohol Conc.Hydrochloric acid Ethyl chloride
Example – 2: (Preparation of isopropyl chloride (2-Chloropropane) from isopropyl alcohol (Propan-2-ol):
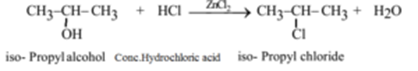
Example – 3: (Preparation of tert- Butyl chloride (2-Chloro-2-methylpropane) from tert- Butyl alcohol (2-Methylpropan-2-ol):

Preparation of Alkyl Bromides:
General Reaction:
When alcohol is treated with concentrated hydrobromic acid (NaBr + H2SO4) alkyl bromide is obtained.
R–OH + HBr ![]() R–Br + H2O
R–Br + H2O
Alcohol Hydrobromic acid Alkyl chloride
Example – 1: Preparation of ethyl bromide (Bromoethane) from ethyl alcohol (Ethanol):
C2H5OH + HBr ![]() C2H5Br + H2O
C2H5Br + H2O
Ethyl alcohol Hydrobromic acid Ethyl bromide
Example – 2: (Preparation of isopropyl bromide (2-Bromopropane) from isopropyl alcohol (Propan-2-ol):

Example – 3: (Preparation of tert- Butyl bromide (2-Bromo-2-methylpropane) from tert- Butyl alcohol (2-Methylpropan-2-ol):

Preparation of Alkyl Iodides:
General Reaction:
When alcohol is refluxed with Potassium or sodium iodide with 95% phosphoric acid and heated, alkyl iodide is obtained.
R–OH + HI ![]() R–I + H2O
R–I + H2O
Alcohol Hydroiodic acid Alkyl iodide
Example – 1: Preparation of ethyl iodide (Iodoethane) from ethyl alcohol (Ethanol):
C2H5OH + HI ![]() C2H5I + H2O
C2H5I + H2O
Ethyl alcohol Hydroiodic acid Ethyl iodide
Example – 2: (Preparation of isopropyl iodide (2-Iodopropane) from isopropyl alcohol (Propan-2-ol):

By Action of Phosphorous Halides:
- This method is suitable for preparation of primary and secondary alkyl halides.
- A good yield of tertiary alkyl halides cannot be obtained by this method.
- The reaction of an alcohol with PX3 does not involve the formation of carbocation and usually occurs without rearrangement of the carbon skeleton.
Preparation of Alkyl Chlorides Using PCl3:
General Reaction:
When alcohol is treated with phosphorous trichloride, alkyl chloride and phosphorous acid are obtained.
3 R-OH + PCl3 ![]() 3 R-Cl + H3PO3
3 R-Cl + H3PO3
Alcohol phosphorous trihalide alkyl halide phosphorous acid
Example – 1: Preparation of ethyl chloride (Chloroethane) from ethyl alcohol (Ethanol):
3C2H5OH + PCl3 ![]() 3C2H5Cl + H3PO3
3C2H5Cl + H3PO3
Ethyl alcohol Phosphorous trichloride ethyl chloride Phosphorus acid
Both the products are in the liquid state and are separated by fractional distillation.
Example – 2: (Preparation of isopropyl chloride (2-Chloropropane) from isopropyl alcohol (Propan-2-ol):

Preparation of Alkyl Chlorides Using PCl5:
General Reaction:
When alcohol is treated with phosphorous pentachloride, alkyl chloride and phosphoryl chloride (phosphorous oxychloride) are obtained.
ROH + PCl5 ![]() RCl + POCl3 + HCl
RCl + POCl3 + HCl
Alcohol Phosphorous pentachloride Alkyl chloride Phosphoryl chloride Hydrogen chloride
Example – 1: Preparation of ethyl chloride (Chloroethane) from ethyl alcohol (Ethanol):
C2H5OH + PCl5 ![]() C2H5Cl + POCl3 + HCl
C2H5Cl + POCl3 + HCl
Ethyl alcohol Phosphorous pentachloride Ethyl chloride Phosphoryl chloride Hydrogen chloride
Example – 2: (Preparation of isopropyl chloride (2-Chloropropane) from isopropyl alcohol (Propan-2-ol):

Preparation of Alkyl Bromides:
Unlike PCl3, the compounds PBr3 and PI3 are not stable and hence they are to be prepared when they are to be used by treating bromine and iodine with red phosphorous. Compounds PBr5 and PI5 do not exist.
Alkyl bromides are prepared by the action of bromine, in presence of red phosphorus, on alcohols. Phosphorus bromide PBr3, which is unstable is formed as intermediates in the reaction.
3 R-OH + PBr3 ![]() 3 R-Br + H3PO3
3 R-Br + H3PO3
Alcohol phosphorous tribromide alkyl bromide phosphorous acid
Example – 1: Preparation of ethyl chloride (Chloroethane) from ethyl alcohol (Ethanol):
3C2H5OH + PBr3 ![]() 3C2H5Br + H3PO3
3C2H5Br + H3PO3
Ethyl alcohol Phosphorous tribromide Ethyl bromide Phosphorus acid
Preparation of Alkyl Iodides:
Alkyl iodides are prepared by the action of iodine, in presence of red phosphorus, on alcohols. Phosphorus iodide PBr3, which is unstable is formed as intermediates in the reaction.
3 R-OH + PI3 ![]() 3 R-I + H3PO3
3 R-I + H3PO3
Alcohol phosphorous triiodide alkyl iodide phosphorous acid
Example – 1: Preparation of ethyl iodide (Iodoethane) from ethyl alcohol (Ethanol):
3C2H5OH + PI3 ![]() 3C2H5I + H3PO3
3C2H5I + H3PO3
Ethyl alcohol Phosphorous triiodide ethyl iodide Phosphorus acid
By Action of Thionyl Chloride:
This reaction is also known as Darzen’s Procedure.
General Reaction:
When alcohol is refluxed with thionyl chloride, alkyl chloride, sulphur dioxide and hydrogen chloride are obtained. Better yield is obtained if pyridine is added in a small amount.
ROH + SOCl2 ![]() RCl + SO2 ↑ + HCl ↑
RCl + SO2 ↑ + HCl ↑
Alcohol Thionyl chloride Alkyl chloride
Example – 1: Preparation of ethyl chloride (Chloroethane) from ethyl alcohol (Ethanol):
C2H5OH + SOCl2 ![]() C2H5Cl + SO2 ↑ + HCl ↑
C2H5Cl + SO2 ↑ + HCl ↑
Ethyl alcohol Thionyl chloride Ethyl chloride
Example – 2: (Preparation of isopropyl chloride (2-Chloropropane) from isopropyl alcohol (Propan-2-ol):

The use of thionyl chloride for preparation of alkyl chlorides is most convenient because the other products of reaction (SO2 and HCI) being gases go off (or can be expelled during distillation very easily). and hence the method requires no special purification/separation.
C5H11OH on bromination gives compound C5H11Br. Suggest all possible structures of alcohol and bromide.

