Science > Chemistry > Organic Chemistry > Halogen Derivatives of Alkanes > Preparation of Alkyl halides From Alkenes
In the last article, we have studied the preparation of alkyl halides from alkanes. In this article, we shall study the preparation of alkyl halides from alkenes (olefins).
The Action of HX on Alkenes:
- It is an electrophilic addition reaction.
- Carbonium ion is formed as an intermediate.
- This method is more useful to prepare secondary and tertiary alkyl halides.
- For unsymmetric alkene, Markownikoff’s rule should be applied.
- Hydrogen chloride and hydrogen iodide add according to Markownikoff’s rule.
- The addition of hydrogen bromide takes place according to Markownikoff’s rule only when the reaction is carried out in the total absence of light or oxygen or peroxides. In the presence of any one of these agents the addition of HBr takes place in exactly the reverse way and is called Peroxide e1ffect or anti-Markownikoff addition or Kharasch effect or Kharasch-Mayo effect.
General Reaction
R –CH=CH2 + H– X → R –CH2–CH2X OR R –CH2X–CH2
Alkene Hydrogen halide Alkyl halide
Conversion of -C=C- (Alkenes) into -X (Alkyl halides)
Order of Reactivity for Halogen acids:
HI > HBr > HCI
Preparation of Alkyl Halides From Symmetrical Alkenes:
R –CH=CH–R + H–X → R –CH2–CHX–R
Symmetric alkene Hydrogen halide alkyl halide
Preparation of Alkyl Chlorides / Alkyl Bromides / Alkyl Iodides:
R –CH=CH–R + H–Cl → R –CH2–CHCl–R
Symmetric alkene Hydrogen chloride alkyl chloride
Example – 1: Preparation of ethyl chloride (Chloroethane) from Ethylene (Ethene):
H2C=CH2 + H–Cl → CH2–CH2Cl
Ethylene hydrogen chloride Ethyl chloride
Example – 2: Preparation of ethyl bromide (Bromoethane) from Ethylene (Ethane):
H2C=CH2 + H–Br → CH2–CH2Br
Ethylene Hydrogen bromide Ethyl bromide
Example – 3: Preparation of Ethyl iodide (iodoethane) from Ethylene (Ethane):
H2C=CH2 + H–I → CH2–CH2I
Ethylene Hydrogen iodide Ethyl iodide
Example – 4: Preparation of sec-butyl chloride (2-Chlrobutane) from Butylene (But-2-ene):
CH3 –CH=CH–CH3 + H–Cl → CH3 –CH2–CH2Cl–CH3
butylene Hydrogen chloride sec-butyl chloride
Example – 5: Preparation of sec-butyl bromide (2-Bromobutane) from β-butylene (But-2-ene):
CH3 –CH=CH–CH3 + H–Br → CH3 –CH2–CH2Br–CH3
butylene Hydrogen bromide sec-butyl bromide
Preparation of Alkyl Halides From Unsymmetrical Alkenes:
R –CH=CH–R’ + H–X → R –CH2–CHX–R’ or R –CHX–CH2–R’
Unsymmetric alkene Hydrogen halide alkyl halide
Markownikoff’s Rule:
When an unsymmetrical reagent (like HBr) is added to an unsymmetrical alkene, (in the total absence of oxygen and peroxide and light) then the negative part of the reagent gets attached to that unsaturated carbon atom which carries less number of hydrogen atoms.
This behaviour is explained by 1,2-hydride shift to attain greater stability of cation.
Example – 6: Preparation of isopropyl bromide (2-Bromopropane) from propene:
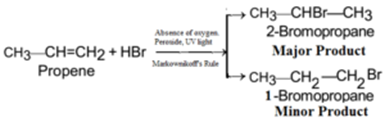
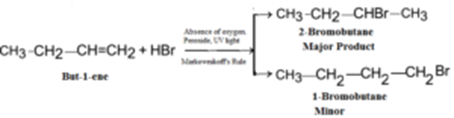
Example – 7: Preparation of sec-butyl bromide (2-Bromobutane) from α-butylene (But-1-ene):
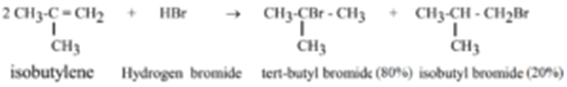
Example – 8: Preparation of tert-butyl bromide (2-Bromo-2-methylpropane) from iso-butylene (2-Methylpropene):
Example – 9: Preparation of 2-Bromo-2-methylbutane from 3-Methyl-but-ene:

The common name of 3-Methyl-but-1-ene is alpha-isoamylene. The above reaction is an example of the 1,2-hydride shift to attain greater stability of cation.
Anti-Markownikoff’s Rule OR Kharasch Effect OR Kharasch Mayo Effect OR Peroxide Effect:
When an unsymmetrical reagent (like HBr) is added to an unsymmetrical alkene in presence of oxygen or peroxide or light then the negative part of the reagent gets attached to that unsaturated carbon atom which carries more number of hydrogen atoms.
Example – 10: Preparation of n-propyl bromide (1-Bromopropane) from propene:

Example – 11: Preparation of n-butyl bromide (1-Bromobutane) from α-butylene (But-1-ene):

Notes:
- This reaction is a free radical addition and exothermic reaction.
- H-Cl has a bond energy of 103 Kcal/mol which is stronger than H-Br bond energy (87 Kcal/mol). Hence there is no breaking up of the H-Cl bond due to peroxide free radicals.
- H-I has a bond energy of 78 Kcal/mol. It forms free radicals easily but instead of attacking the double bond, the iodine radicals formed combine with each other to form an iodine molecule.