Science > Chemistry > Chemical Kinetics > Arrhenius Equation
In this article, we shall study the factors affecting the rate of a chemical reaction and the Arrhenius equation.
Factors Affecting the Rate Of Reaction:
The Concentration of Reactants:
The number of collisions and hence the activated collisions between the reactant molecules increase with the increase in concentration. According to the collision theory, the rate of a reaction should increase with the increase in the concentration since the rate is directly proportional to the collision frequency.
Pressure:
The number of collisions increases with an increase in the partial pressures of gases. Hence the rate of a reaction involving gaseous reactants increases with an increase in partial pressures. However, it has no effect on reactions involving reactants in liquid or solid phases.
It is important to keep in mind that the partial pressures of reactants can be increased by increasing the pressure of the overall system. However, the partial pressures do not increase when an inert gas or a non-reacting gas is added to the reaction mixture at constant volume.
Temperature:
The average kinetic energy increases with increase in absolute temperature. Hence the number of molecules with energy greater than the threshold energy also increases.
As a result, the number of effective collisions between reactant molecules also increases. Therefore, usually, it is observed that the rate of reaction increases with increase in temperature.
Catalyst:
The catalyst is a substance which changes the rate of a reaction without being consumed or without undergoing any chemical change during the reaction.
In case of reversible reactions, the catalyst lowers the activation energies of both forward and backward reactions to the same extent and helps in attaining the equilibrium quickly.
Some substances may decrease the rate of a reaction. These are generally referred to as negative catalysts or inhibitors. They interfere with the reaction by forming relatively stable complexes, which require more energy to break up. Thus the speed of the reaction is reduced.
Nature of Reactants:
The rate of a reaction depends on the nature of bonding in the reactants. Usually, the ionic compounds react faster than covalent compounds.
The reactions between ionic compounds in water occur very fast as they involve the only exchange of ions, which were already separated in aqueous solutions during their dissolution.
Whereas, the reactions between covalent compounds take place slowly because they require energy for the cleavage of existing bonds.
The Orientation of Reacting Species:
The reaction between the reactants occurs only when they collide in the correct orientation in space. Greater the probability of collisions between the reactants with proper orientation, greater is the rate of reaction.
The orientation of molecules affects the probability factor, p. The simple molecules have more ways of proper orientations to collide. Hence their probability factor is higher than that of complex molecules.
The orientation factor also affects the interaction between reactants and catalysts. For example in case of biological reactions, which are catalyzed by enzymes, the biocatalysts. The enzymes activate the reactant molecules (or substrates) at a particular site on them. These sites are called active sites and have definite shape and size. The size, stereochemistry, and orientation of substrates must be such that they can fit into the active site of the enzyme. Then only the reaction will proceed. This is also known as lock and key mechanism. The enzymes lose their activity upon heating or changing the pH or adding certain chemical reagents. This is due to deformation of the configuration of the active site.
Surface Area of Reactant:
The rate of a reaction increases with increase in the surface area of solid reactant if any used. The surface of a solid can be increased by grinding it to a fine powder.
This is also true with the solid catalysts, which are usually employed in finely powdered form while carrying out a chemical reaction.
The Intensity of Light:
The rate of some photochemical reactions, which occur in presence of light, increases with increase in the intensity of suitable light used.
With the increase in the intensity, the number of photons in light also increases. Hence number of reactant molecules get energy by absorbing more number of photons and undergo a chemical change.
Nature of Solvent:
The solvent may affect the rate in many ways as explained below:
The solvents are used to dissolve the reactants and while doing so they help in providing more interactive surface between reactant molecules which may be otherwise in different phases or strongly bonded in the solid phase.
Usually, solvents help in breaking the cohesive forces between ions or molecules in the solid state. The polar molecules tend to dissolve more in polar solvents with more dielectric constants and react faster in them. Whereas nonpolar molecules prefer nonpolar solvents.
In case of diffusion controlled reactions, the viscosity of the solvent plays a major role. The rate decreases with increase in the viscosity of the solvent.
Effect of Change of Temperature on the Rate of Reaction:
The average kinetic energy increases with increase in absolute temperature. Hence the number of molecules with energy greater than the threshold energy also increases.
As a result, the number of effective collisions between reactant molecules also increases. Therefore, usually, it is observed that the rate of reaction increases with increase in temperature.
The two distribution graphs are shown below for a lower temperature T1 and a higher temperature T2. The area under each curve represents the total number of molecules whose energies fall within the particular range.
The shaded regions indicate the number of molecules which are sufficiently energetic to meet the requirements dictated by the two values of Ea that are shown.
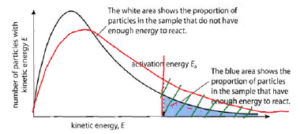
It is clear from these graphs that the fraction of molecules whose kinetic energy exceeds the activation energy increases quite rapidly as the temperature is raised. This the reason that virtually all chemical reactions (and all elementary reactions) proceed more rapidly at higher temperatures.
Arrhenius Equation:
Arrhenius came up with an equation that demonstrated that rate constants of different kinds of chemical reactions varied with temperature. This equation indicates a rate constant that has a proportional relationship with temperature. For example, as the rate constant increases, the temperature of the chemical reaction generally also increases. The result is given below:
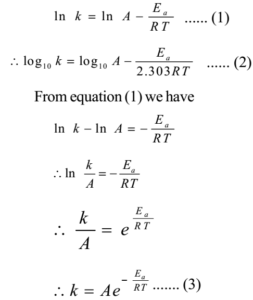
Where k is rate constant, Ea the activation energy, T the absolute temperature of the reaction and R is universal gas constant. A is called frequency factor or pre-exponential factor and proportional to the frequency of collisions between reacting molecules. A is independent of the absolute temperature T.
Equations (1), (2) and (3) are different forms of Arrhenius equation. A and Ea are called Arrhenius parameters.
Arrhenius Equation and Temperature Variation:
The relation between rate constant k and the absolute temperature T of the reaction is given by
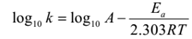
For two different temperatures say T1 and T2 we have

Determination of Activation Energy:
We have

By knowing Values of K1 and K2 at temperatures T1 and T2 using experiments, the value of activation energy can be calculated.