Science > Chemistry > Chemical Kinetics > Collision Theory
The occurrence of a bimolecular chemical reaction can be explained on the basis of collision theory.
The Collision of Reacting Molecules:
Consider a bimolecular general reaction
A + B → C
In order for a chemical reaction to take place, the molecules of reactants A and B must collide. The rate of a chemical reaction depends on the rate of collision between the molecules. As the concentration and temperature increase, the rate of reaction also increases.
The Energy of Activation:
The collision between the molecules in a chemical reaction provides the kinetic energy needed to break the necessary bonds so that new bonds can be formed. If the kinetic energy is not sufficient the bond between the reactants will not be broken and thus new bonds will not be formed. This required energy is called activation energy.
The activation energy (Eact) is defined as the minimum kinetic energy required for the molecular collision to lead to the reaction.
Thus reaction to take place the kinetic energy of colliding molecules should be greater than the activation energy.
The Orientation of Reacting Molecules:
The minimum kinetic energy (energy of activation) does not mean successful collision leading to the reaction.
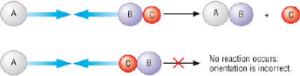
For successful collision leading to the reaction, the colliding molecules must be so oriented relative to each other that the group reacting or bonds to be shifted are relatively close. This criterion is not essential for simple molecules but it becomes very much essential for complex molecules.
Potential Energy Barrier:
Consider a bimolecular general reaction
B + AC → BA + C
During the collision the electron distribution about the three nuclei A, B and C changes in such a way that the new bond B-A strengthens at the same time the old bond A-C weakens. A stage is reached when all the three nuclei are weakly linked together. This state is called activated complex or transition state.
B + AC → B—-A —-C
The transition state is an unstable transitory complex of the highest energy state through which the reactants must pass on the way to products. It decomposes spontaneously to form products.
To achieve this configuration, atoms require energy to overcome the repulsion between B and AC that have filled shells of electrons. This energy comes from kinetic energy due to the collision of reacting molecules and gets converted into potential energy in the activated complex. Thus transition state has the highest energy state. In an energy profile diagram (E.P.D.), the highest energy point (Peak) corresponds to the transition state.
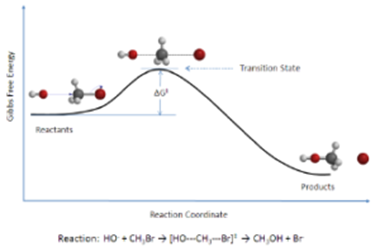
Thus reactant molecules have to climb up the barrier to get converted into products.
Mathematical Treatment to Collision Theory:
Consider a bimolecular general reaction
B + AC → BA + C
It is a second-order reaction. Hence the rate of collision is given by
Rate of collision = Z [AC] [B]
Where Z = frequency of collision.
The reaction rate is given by
Rate of reaction = P x f x Rate of collision
Where P is the fraction of collisions with proper orientations of colliding molecules. f is a fraction of molecules with sufficient kinetic energy.
Rate of reaction = P x f x Z [AC] [B] …… (1)
Burt the rate of second-order reaction is given by
Rate of reaction = k [AC] [B] …… (2)
From (1) and (2) we have
k = P.f.Z
By Arrhenius equation we have

Where A = P.Z and called as frequency factor or pre-exponential factor.