Science > Chemistry > Atomic Structure > Problems on Calculation of Number of Electrons, Protons, and Neutrons In this article, we shall study to solve problems on the calculations of the number of electrons, protons, and neutrons in atoms, molecules, and species. Example 01: Calculate the charge and mass of 1 mole of electrons. Solution: […]
Tag: Physical chemistry
Science > Chemistry > Atomic Structure > Problems Based on Atomic Number, Mass Number, and Neutron Number In this article, we shall study to solve problems based on the calculation of atomic number, atomic mass number, and neutron number Atomic number (Z) : The number of protons (positive charge) present in the nucleus of an […]
Science > Chemistry > States of Matter > Charle’s Law Mathematical relationships between volume, pressure, and temperature of a given mass of gas are referred to as Gas laws. In this article. we shall study Pressure-Temperature relation or Gay-Lussac’s law. Gay-Lussac’s Law: Statement: At constant volume the pressure of a given mass of a gas […]
Properties of Substance

Science > Chemistry > Introduction to Chemistry > Properties of Substance All matter has physical and chemical properties. Extensive properties are those properties of a substance which depend on the amount of substance. They vary with the amount of the substance. Examples: Mass, weight, and volume. Intensive properties are those properties of a substance which do not depend […]
Arrhenius Equation
Science > Chemistry > Chemical Kinetics > Arrhenius Equation In this article, we shall study the factors affecting the rate of a chemical reaction and the Arrhenius equation. Factors Affecting the Rate Of Reaction: The Concentration of Reactants: The number of collisions and hence the activated collisions between the reactant molecules increase with the increase […]
Collision Theory
Science > Chemistry > Chemical Kinetics > Collision Theory The occurrence of a bimolecular chemical reaction can be explained on the basis of collision theory. The Collision of Reacting Molecules: Consider a bimolecular general reaction A + B → C In order for a chemical reaction to take place, the molecules of reactants A and […]
Science > Chemistry > Chemical Kinetics > Molecularity of Reaction and Catalysis In this article, we shall study the molecularity of reaction and catalysis. The Concept of Elementary Reactions: Many reactions that follow a simple rate law are actually taking place in series of steps. These reactions are called complex reactions. Each step in a […]
Science > Chemistry > Chemical Kinetics > Rate of Zero Order Reaction In this article, we shall study the analytical treatment to the zero-order reaction, and the rate of zero-order reaction. Order of Reaction: The overall order of the reaction is defined as the sum of the exponents to which the concentration terms in the […]
First Order Reaction
Science > Chemistry > Chemical Kinetics > Rate of First Order Reaction In this article, we shall study the order of reaction and the analytical treatment to the first-order reaction, and the rate of the first-order reaction. Order of Reaction: The overall order of the reaction is defined as the sum of the exponents to […]
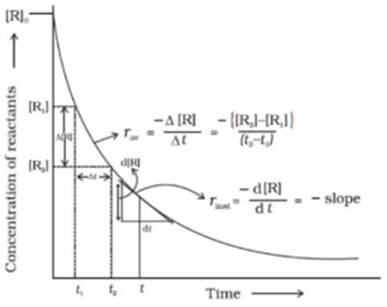
Science > Chemistry > Chemical Kinetics > Introduction to Chemical Kinetics In this article, we shall discuss the concept of chemical kinetics, rate of reactions, and types of reactions on the basis of their rates. Rate of Reaction: The branch of chemistry, which deals with the rate of chemical reactions, the factors affecting the rate […]