Science > Chemistry > States of Matter > Charle’s Law
Mathematical relationships between volume, pressure, and temperature of a given mass of gas are referred to as Gas laws. In this article. we shall study Pressure-Temperature relation or Gay-Lussac’s law.
Gay-Lussac’s Law:
Statement:
At constant volume the pressure of a given mass of a gas increases or decreases by 1/273 of its pressure at 0oC for every degree rise or fall in temperature.
Explanation:
Let Po be the volume of a gas at 0 °C, Let this gas be heated through t °C, Let Pt be the volume of the gas at t °C. then,
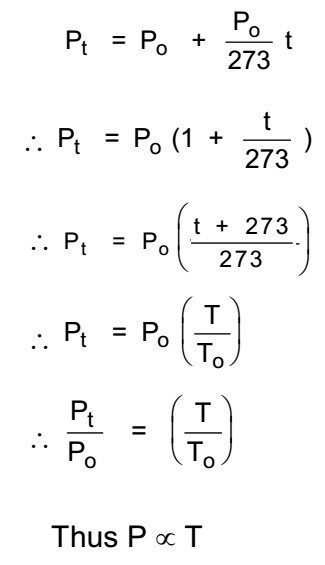
Alternate Statement of Gay-Lussac’s law:
Thus at constant volume, the pressure of the certain mass of enclosed gas is directly proportional to the absolute temperature of the gas.
In general
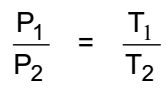
This relation is called the pressure-temperature relation.
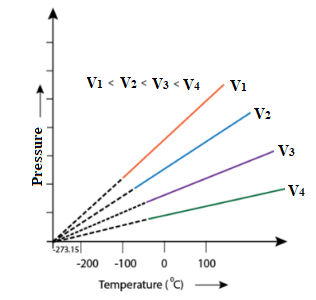
Graphical Representation:
A graph is drawn by taking the absolute temperature on the x-axis and pressure on the y-axis. The graph is as follows. This graph is also known as a P-T diagram.
Numerical Problems:
Example 01:
A steel tank contains air at a pressure of 15 bar at 20 oC. The tank is provided with a safety valve which can withstand a pressure of 35 bar. Calculate the temperature to which the tank can be safely heated.
Solution:
Given: Initial pressure P1 = 15 bar, Initial temperature = 20 oC = 20 + 273 = 293 K, Final pressure P2 = 35 bar
To Find: Temperature up to which tank can be heated = T2 =?
By Gay-Lussac’s Law

T2 = (P2 x T1)/P1 = (35 x 293)/15 = 683.67 K
T2 = 683.67 – 273.15 = 410.15 oC
Ans: The tank can be heated up to 410.15 oC
Example 02:
An iron tank contains helium at a pressure of 2.5 atm at 25 oC. The tank can withstand a maximum pressure of 10 atm. The building in which the tank has been installed catches fire. Predict whether the tank will blow up first or melt if the melting point of iron is 1535 oC.
Solution:
Given: Initial pressure P1 = 2.5 atm, Initial temperature = 25 oC = 25 + 273 = 298 K, Melting point of iron = T2 = 1535 oC = 1535 + 273 = 1808 K
To Find: Final pressure = P2 =?
By Gay-Lussac’s Law

P2 = (T2 x P1)/T1 = (1808 x 2.5)/298 = 15.16 atm
The pressure at the melting point is 15.16 atm, which is much more than the maximum pressure that the tank can withstand 10 atm. Hence the tank will blow up before reaching the melting point.
Ans: The tank will blow up before reaching the melting point.